21
Venous Thromboembolic Disease and Vena Cava Filters
 Epidemiology
Epidemiology
Lower-extremity deep-vein thrombosis (DVT) is a common condition that is seen in 34% of unselected medical patients and in 60% of unselected surgical patients by autopsy series.1 Of the patients presenting with lower-extremity DVT, 35% to 51% have evidence of pulmonary embolism (PE) by ventilation perfusion scan, indicating that these two disease processes should be thought of as a continuum of the same process rather than as two separate conditions.2–4 PE has been stated to be the third leading cause of death in this country5 and is the third most common disease of the cardiovascular system, after myocardial infarction and stroke.6
The incidence of PE in the United States has been estimated to be as high as 750,000 to 900,000 cases per year, with a mortality of 120,000 to 150,000 cases annually.7,8 Untreated, proximal DVT or PE has a 30.5 to 50% chance of developing into recurrent PE, with a mortality rate of 18 to 26%.9,11 Of pulmonary emboli, 85 to 95% arise from the iliofemoral veins,12,13 with most of the other 5 to 15% arising from thrombi in the vena cava, ovarian veins, right atrium, or upper extremities.12,14,15
Asymptomatic thrombus formation in the veins of the calf, particularly the soleal sinuses, is probably a normal process that occurs throughout life in all persons. It is only when thrombus is not lysed by the body but rather propagates to a large central vein that a pathological condition exists. The propensity for progression to this pathological state has been observed for several groups of hospitalized patients, including those with recent surgery or trauma, heart disease, neoplastic disease, and systemic diseases.6 Ultrasound examinations of the lower extremities often demonstrate asymptomatic DVT in many of these patients. For example, routine ultrasound examination of the lower-extremity veins in 542 asymptomatic intensive care unit (ICU) patients demonstrated previously unsuspected DVT in 11.4% of patients.16 Unsuspected proximal DVT has been reported in 13 to 16% of patients presenting with acute hip fracture in two series using ultrasound;17,18 the incidence of proximal DVT has been reported as even higher (32%) in one series using ascending venography.19 Recently, the incidence of postoperative DVT in 50 patients who underwent abdominal aortic aneurysm repair was reported as 18%, although involvement was limited to the calf veins in 78% of these patients.20 Unfortunately, in 70% of patients, the diagnosis of PE is not made before the patient dies.21
 Diagnosis of DVT
Diagnosis of DVT
Before the arrival of real-time ultrasound, the standard method of documenting lower-extremity DVT was contrast venography (Fig. 21-1). Contrast venography possesses several disadvantages relative to ultrasound that have led to replacement of venography by ultrasound as the initial imaging method for diagnosing this disease. First, contrast venography cannot be performed at the bedside. Patients must be transported to the radiology department to be evaluated by this technique and additionally must be able to tolerate the changes in position required for optimal performance of this examination. Also, contrast introduced into the lower-extremity veins has been associated with an increased incidence of postvenography thrombophlebitis. This condition is suspected to result from osmotic injury to the venous endothelium, which causes local inflammation and pain,22,23 and is seen in up to 24% of patients studied with full-strength contrast medium.22 Some authors believe that contrast venography occasionally can result in occlusive thrombosis of the vein.24 It also has been reported that up to 4.6% of attempts to perform lower-extremity venography are unsuccessful because of an inability to cannulate a suitable vein or because of inadequate opacification of the femoral or iliac veins.25 Contrast venography is still useful in patients who undergo nondiagnostic or technically inadequate examinations (e.g., obese patients).
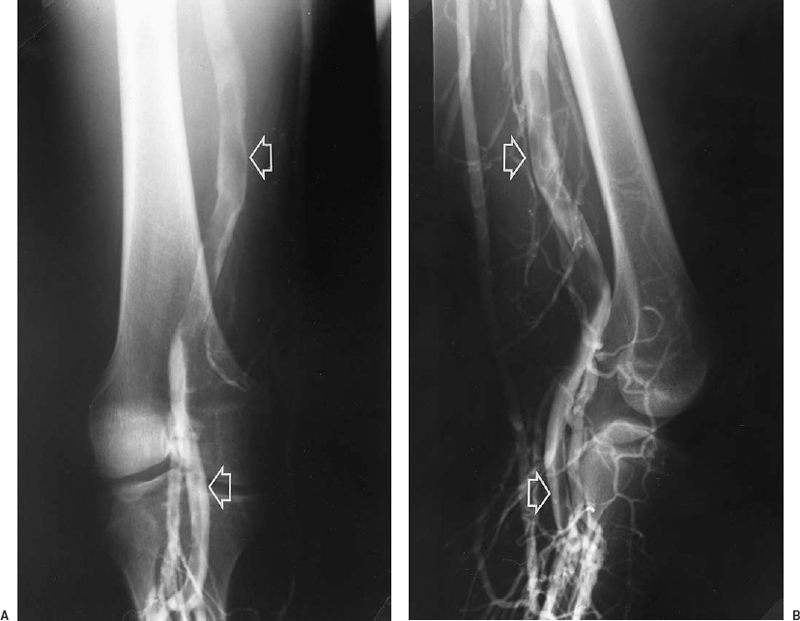
FIGURE 21-1. Anterior (A) and lateral (B) views from a contrast venogram of the left lower extremity, showing an intraluminal filling defect in the popliteal and superficial femoral veins (arrows). A filling defect is the most specific sign of deep vein thrombosis on contrast venograms.
Other noninvasive imaging methods for evaluating lower-extremity veins include impedance plethysmography (IPG), radionuclide venography, magnetic resonance venography, and ultrasound. IPG is inaccurate in assessing the popliteal and calf veins and requires patient cooperation to prevent false-positive results.26,27 Radionuclide venography is inaccurate and insensitive to isolated thrombus below the knee and to nonocclusive thrombus above the knee.28 Iodine-125 labeled fibrinogen leg scanning is insensitive to thrombus above the midthigh, is expensive, and requires 24 to 48 hr to complete.28 Magnetic resonance venography has demonstrated a sensitivity of 90 to 100% and a specificity of 95 to 100% in four published series with a total of 325 patients.29–32 At present, magnetic resonance angiography does not have a role in the diagnosis of DVT in most patients because of its lack of demonstrated benefit over ultrasound, which is less expensive. D-dimer assays of whole blood have demonstrated excellent negative predictive value and may be useful in excluding DVT without imaging tests; however, the specificity of an elevated D-dimer level is poor, and this test cannot reliably establish the diagnosis of DVT.33
Ultrasound is the imaging method of choice for the initial evaluation of DVT. The standard ultrasound lower-extremity examination for deep venous thrombosis includes real-time compression of the vein (Fig. 21-2), Doppler analysis at rest and during compression of the calf, and color Doppler assessment of the vein. Examination of the calf veins often is performed if proximal thrombi are not detected, and reflux studies often are done if all the preceding are normal and there is suspicion of chronic venous insufficiency. Ultrasound also can allow the diagnosis of chronic venous changes to be made by visualization of thickening of the vein wall.
Review of the literature since the advent of venous ultra-sound reveals 20 studies in which compression ultrasound and Doppler interrogation of the veins or compression ultrasound alone was compared with contrast venography for the detection of symptomatic DVT.34 Some controlled studies included venograms and ultrasound examinations performed on consecutive patients, whereas others had venographic confirmation performed only for abnormal ultrasound studies; however, the cumulative sensitivity of the technique for proximal thrombus (in the iliac, femoral, or popliteal veins) is 97% and the specificity 96%, for an accuracy of 97% in a total of 1,660 patients who had venographic control of ultrasound studies. Although specific examination of calf veins was not performed in these series, inclusion of isolated calf clot accounting for false-negative ultrasound studies yields only eight additional false-negative examinations in a total of 754 patients with symptomatic lower extremity DVT, which reduces sensitivity to 94%.34 Although examination of calf veins can be performed accurately by ultrasound, to do so will lengthen examination time significantly and is operator dependent. It is also of dubious clinical use because patients with clinically significant pulmonary embolus usually do not have thrombi limited to the calf veins, and when they do, it is likely that emboli arose proximal to the calf veins.35,36 Rather than treating calf thrombus detected on ultrasound, repeat studies at 3- to 5-day intervals can search for the estimated 5 to 20% of clots that will propagate upward into the femoropopliteal system.35,36 Interobserver variability in the interpretation of lower-extremity venograms has been reported to be 10%,37 and venography has been reported to be as low as 89% sensitive and 97% specific compared with autopsy findings, including calf vein thrombosis.38 The overall clinical performance of ultrasound in assessing lower extremity DVT appears to be at least equal to that of contrast venography.
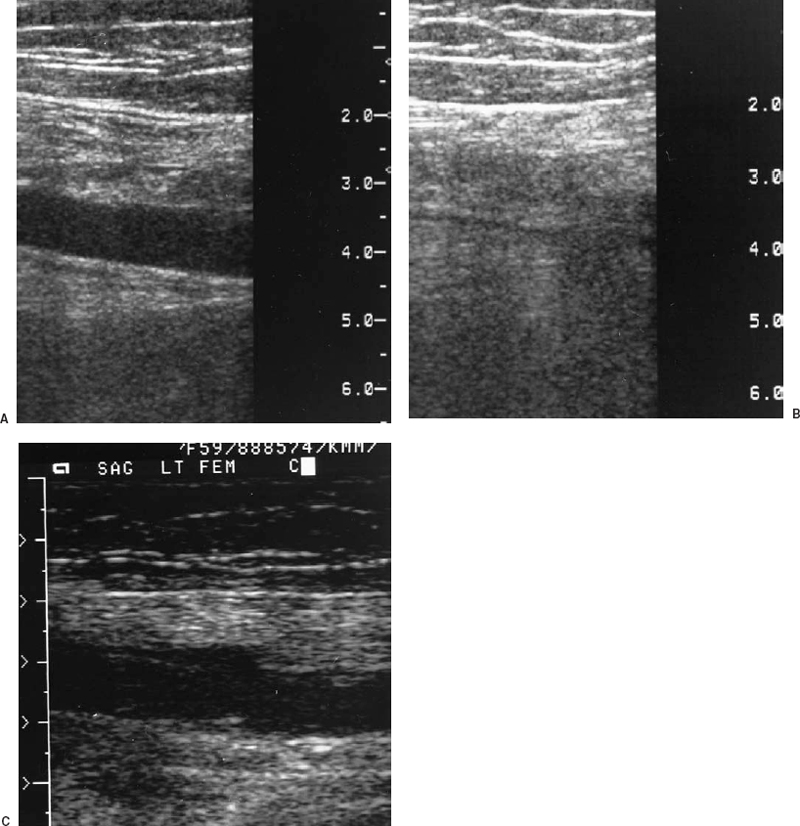
FIGURE 21-2. A: Sagittal view of the superficial femoral vein by real-time ultrasound. B: After compression, the vein collapses and the lumen is obliterated, indicating absence of thrombus. C: Sagittal compression view of the superficial femoral vein from another patient demonstrates lack of compression, consistent with thrombosis of the vein.
 Diagnosis of PE
Diagnosis of PE
Pulmonary angiography is the gold standard for the evaluation of PE; however, this test is invasive, and major complications occur in 3.2%, and 0.2% of patients die.39 Most deaths occur in patients with elevated pulmonary artery pressures.39 Pulmonary angiography is also expensive. Although radioisotope lung scanning is established as the best screening test for patients with suspected PE and is highly specific in the proper clinical setting, this test suffers from lack of sensitivity. The Prospective Investigation of Pulmonary Embolism Diagnosis (PIOPED) investigators noted that of 755 patients undergoing pulmonary angiography and radioisotope lung scanning, only 41% of patients with angiographically proved pulmonary embolus had high-probability ventilation–perfusion scans.40 The incidence of angiographically proved pulmonary embolus in those patients with low-probability ventilation-perfusion scans was 16%.41
Noninvasive tests have been proposed to improve the sensitivity of ventilation/perfusion scanning for pulmonary embolus in hopes of obviating pulmonary angiography. Magnetic resonance angiography has been used for the diagnosis of acute and chronic PE with mixed results.40,42,43 These studies demonstrate a sensitivity of 85% to 90% for emboli greater than 1 cm in diameter,40,41,44 and a specificity of 77%.42,44 Insensitivity for peripheral emboli has been noted,40 and the role of magnetic resonance imaging in the diagnosis of PE is therefore limited. Quantitative D-dimer levels possess excellent negative predictive value, although specificity is low.45
Ultrasound of the lower-extremity veins has a limited role in the evaluation of the patient with suspected PE. Previous work has demonstrated a 29% incidence of negative bilateral lower-extremity venograms in patients with proven PE by pulmonary angiography.46 Ultrasound would be expected to be similarly insensitive and therefore should not be used to exclude pulmonary embolus. Radionuclide ventilation–perfusion scanning is the initial recommended evaluation in patients with suspected PE; however, in patients with low- or indeterminate-probability ventilation–perfusion scans, ultrasound represents a noninvasive alternative test.47 In one report, 15% of patients with this presentation will demonstrate DVT on ultrasound examination.48 Although it may appear to increase costs to incorporate lower-extremity ultrasound into the workup of PE, this report noted a decrease of 9% in overall costs by avoiding pulmonary angiograms in some patients.49 In a second report, 19% of patients with low- or intermediate-probability ventilation–perfusion scans had abnormal ultrasound studies.50 Interestingly, 21% of patients with normal ventilation–perfusion scans were noted to have lower-extremity thrombus in this study.50 As noted, if lower-extremity ultrasound is negative, thromboembolic disease cannot confidently be excluded and further evaluation with pulmonary angiography is necessary.
 Medical Management of DVT and PE
Medical Management of DVT and PE
Anticoagulation is the standard treatment for pulmonary deep venous thromboembolic disease in most patients. This is usually achieved by beginning intravenous heparin and oral coumadin simultaneously, with maintenance of intravenous anticoagulation for 5 days to allow for depletion of circulating vitamin K–dependent clotting factors II, V, VII, IX, and X.51–53 Low-molecular-weight heparin, which has a long half-life and can be administered subcutaneously once daily, has been shown to be at least equally effective as continuous intravenous unfractionated heparin in preventing pulmonary embolus in patients with proximal deep venous thrombi, with a lower incidence of bleeding complications.54
Thrombolysis has been used in selective patients with DVT and PE. In general, thrombolysis in the lower extremities is reserved for patients with phlegmasia cerulea dolens, or painful swelling with cyanosis. These patients are at risk for venous gangrene and systemic hypotension; 25 to 32% of patients die.55,56 Clinical improvement with catheter-directed thrombolysis has been reported in this condition.57 Recently, less selective criteria for thrombolytic therapy in patients with lower-extremity DVT have been applied. In one series, 21 consecutive patients with iliofemoral DVT underwent catheter- directed thrombolysis.58 Technical and clinical success was achieved in 85%, with 92% 3-month patency (11 of 12) by ultra-sound.58 No major complications were seen. No control group was present, only a few patients were treated, and follow-up was brief.58 It should be noted that the doses of urokinase (Abbokinase, Abbott Laboratories, North Chicago, IL) used to treat acute DVT are high,58 and further data are needed. There is currently an ongoing registry of patients treated in this manner. In a preliminary report from this registry, complete or partial success of thrombolysis was achieved in 48% and 38% of 73 limbs, respectively.59 The mean urokinase dose for patients treated with intrathrombic infusion was 7.4 million units.59 Technical success was greatest in patients who received intrathrombic infusion and in those with iliac vein involvement.59 Stents were used in 47% for abnormalities in iliac veins after thrombolysis.59 Major bleeding complications were observed inseven (10%) patients and remainaconcern,59 especially because the effectiveness of this therapy in reducing the incidence of postphlebitic syndrome will not be known for many years. A decision analysis of the best treatment strategy for DVT based on patients’ responses to a questionnaire regarding the relative value of potential outcomes, including postphlebitic syndrome, intracerebral hemorrhage, and death, found that no patient surveyed was willing to incur the risk of thrombolysis to reduce the risk of postphlebitic syndrome.60 At present, thrombolysis for DVT should be reserved for patients with thrombosis that includes the iliac vein or inferior vena cava (IVC), whose symptoms are severe, who have reasonably long life expectancies, and no contraindication to thrombolysis. Conversely, most patients with spontaneous upper-extremity thrombosis (Paget-Schroetter syndrome) are believed to experience optimal long-term outcome by undergoing thrombolysis in the acute period.61
 History of Venous Interruption
History of Venous Interruption
Virchow’s triad was originally postulated in 1860.62 Just 8 years later, mechanical obstruction as a method to prevent lower-extremity thrombi from traveling to the lungs was conceived.63 Homans recognized in 1944 that most symptomatic pulmonary emboli arise from the lower-extremity and pelvic veins and that these proximal thrombi usually originate in the deep veins of the calf.63 Homans, Debakey, and Ochsner advocated early femoral interruption in patients who had experienced PE.63 Ligation of the IVC became popular because of the venous stasis sequelae of common femoral vein ligation.64,65 Although mortality rates for IVC ligation as low as 2% were reported,66 most series had mortality rates in the 8 to 15% range.67–71 Immediate and severe leg swelling remained a frequent debilitating sequela, occurring in 10 to 16% of patients.68,71 Because heparin did not become available for clinical use until 193572 and warfarin was not synthesized and available for widespread clinical use until 1948,73,74 the use of anticoagulation paralleled vein interruption in the treatment of this disease process.
Methods of partial interruption of the IVC were developed in the late 1960s in an attempt to preserve flow through the IVC while trapping or filtering out potentially harmful emboli. These methods include suture plication75 and various designs of clips used to narrow the lumen of the IVC (Moretz) or compartmentalize the lumen into several smaller channels (Miles, Adams-DeWeese) (Fig. 21-3).71,75 Comparison of partial versus complete interruption of the IVC demonstrated similar procedure-related mortality and recurrent PE rates but a significantly lower incidence of lower-extremity morbidity in the patients treated with partial caval interruption (46 versus 76%).67 Clips and suture plication of the vena cava were associated with 30 to 40% incidence of IVC occlusion.67,71 It is interesting to note that IVC ligation was associated with immediate shock in only approximately 5% of patients67 despite causing significant transient hemodynamic alterations in experimental animals.76
In 1967, Mobin-Uddin introduced the first transvenous device for caval interruption, the Mobin-Uddin umbrella (Edwards Laboratories, Santa Ana, CA) (Fig. 21-4).77 This device was placed through the right internal jugular vein after surgical exposure, through a catheter-based carrier system with an inner diameter of 9 mm (27 Fr). The device was deployed in the IVC using fluoroscopic guidance, and the procedures therefore usually were performed in the angiography suite. The jugular approach was used to avoid potential iliocaval thrombus; a femoral carrier for the Mobin-Uddin umbrella did not exist. The superiority of the transvenous method of IVC interruption compared with surgical methods was established by marked improvement in procedure-related mortality, which was negligible for transvenous placement.77–82
The Mobin-Uddin umbrella was designed as an adjunct to anticoagulation in patients who experienced recurrent PE during adequate anticoagulation. Broad use of devices for caval interruptionin patients with contraindications to anticoagulation, currently the most frequent indication for filter placement, was an infrequent indication when the Mobin-Uddin umbrella was introduced. The original version of this device was imperforate, but the design was modified to include multiple holes, which were intended to permit delayed occlusion of the IVC to allow time for collaterals to develop,77 theoretically reducing the risk of hypotension associated with acute caval occlusion.76 As experience with this device accumulated, it became evident that approximately one third of patients maintained patency of the vena cava after umbrella placement, with no increase in the incidence of recurrent PE, which was reported as 3.6% in a review of 2,215 patients.78 The silicone membrane of this device was subsequently heparin bonded in an attempt to improve caval patency rates. The diameter of the Mobin-Uddin umbrella was increased from 23 to 28 mm in the mid-1970s secondary to occasional proximal migration.78
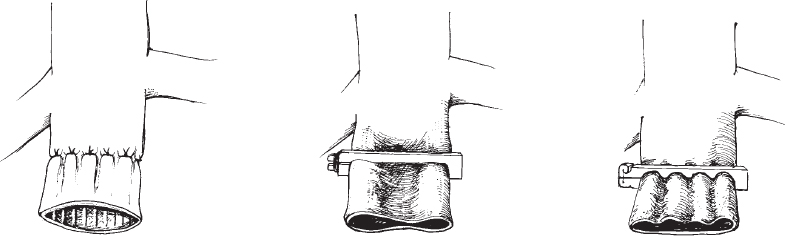
FIGURE 21-3. Line drawings of early surgical approaches to partitioning of the vena cava. A: The “harp-string” grid method of surgical plication popularized by Spencer. B: Moretz vena cava clip. C: Miles vena cava clip. (After Adams JT, Feingold BE, DeWeese JA. Comparative evaluation of ligation and partial interruption of the inferior vena cava. Arch Surg 1971; 03:272–276).
The observation that patients with Mobin-Uddin umbrellas who maintained patency of the IVC had no increased incidence of recurrent PE80,83 and a lower incidence of lower extremity sequelae80,83 led to the subsequent development of new devices designed to maintain caval patency and to the demise of the Mobin-Uddin umbrella, which was removed from the market in 1986. Methods of caval interruption resulting in a high incidence of caval occlusion are performed infrequently in favor of devices designed to preserve patency of the IVC while trapping clinically significant pulmonary emboli. The first such device to be introduced was the Kimray-Greenfield filter, now known as the Greenfield filter.
The Greenfield filter (Boston Scientific, Watertown, MA) was introduced in 1973. Despite the proliferation of many small-profile filter systems, the Greenfield filter remains one of the most popular vena cava filters in use today. Because the original Greenfield had a large profile, it was initially placed by surgical cut-down, usually of the right internal jugular vein, but also occasionally by the right common femoral vein. Percutaneous placement was first described by Tadavarthy in 1984.84
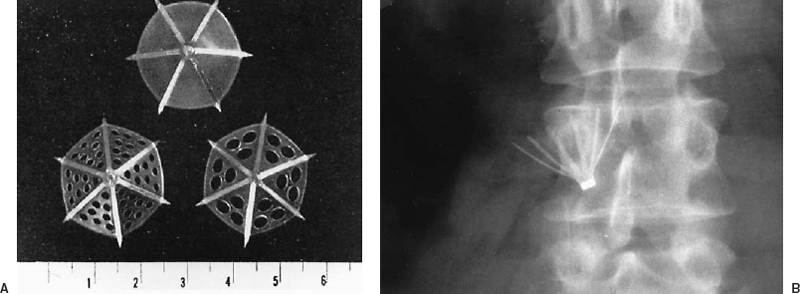
FIGURE 21-4. A: Axial view of three configurations of the Mobin-Uddin umbrella. The initial configuration consisted of six radial struts with a solid silicone membrane. Subsequently, the filter was modified to contain fenestrations of 1.5 mm or 3 mm in the silicone membrane. (Reprinted with permission from Mobin Uddin K, McLean R, Bolooki H, Jude JR. Caval interruption for prevention of pulmonary embolism: long-term results of a new method. Arch Surg 1969;99:711–715). B: A radiograph of a Mobin-Uddin umbrella demonstrates the caudal orientation of the filter apex.
 Indications for Vena Cava Filter Placement
Indications for Vena Cava Filter Placement
DVT or PE and a contraindication to anticoagulation, noted in 38 to 77% of patients, are currently the most frequent indications for vena cava filter placement.85–90 Absolute contraindications to anticoagulation include recent (within 2 months) stroke or neurosurgical procedure, recent major surgery or trauma (within 2 weeks), active internal bleeding, intracranial neoplasm, recent ocular surgery, and heparin-induced thrombocytopenia.91–95 Coumadin is contraindicated in pregnancy because it crosses the placenta and potentially can cause fetal anomalies.94 Relative contraindications include recent trauma (within 2 weeks), hematuria, occult blood in the stools, peptic ulcer disease, pericarditis, bacterial endocarditis, and unstable gait. Less frequent indications for vena cava filter placement include complications of anticoagulation, which occur in 5 to 50% of patients and include bleeding, thrombocytopenia secondary to heparin therapy, and warfarin-induced skin necrosis.91–95 Long-term anticoagulation is associated with 5 to 12% mortality rate.91–95 Complications of anticoagulation are the indication for filter placement in 6 to 17% of patients.85–90 In 3 to 27% of patients, the indication for filter placement is recurrent PE or proximal propagation of lower-extremity thrombus while adequately anticoagulated.85,87–90 High-risk of PE is seen in patients with free-floating iliofemoral or caval thrombus, occuring in 27 to 60% of these patients despite adequate anticoagulation.96–99 These situations also warrant placement of a vena cava filter, ideally as an adjunct to anticoagulation.87
In the era when filters were placed surgically, filter placement was reserved for the most severely affected patients. Patients with recurrent PE constituted a disproportionately large percentage of patients receiving vena cava filters. Although PE is rare when patients are adequately anticoagulated (usually < 5%),10,53 such patients were seen in up to 31% of those who underwent surgical placement of vena cava filters.98 After introduction of the percutaneous method of vena cava filter placement, the mortality of filter placement was recognized to be negligible and clinically significant complications rare. The number of patients having filters placed subsequently increased at most institutions,99 and the threshold for filter placement was lowered. This approach resulted in broadening the indications for vena cava filter placement, including routine use of vena cava filters at some institutions in high-risk trauma patients without DVT or PE or in patients with cancer as primary therapy for DVT or PE so that anticoagulation can be avoided.
The considerations for the treatment or prophylaxis of deep venous thromboembolic disease in patients with advanced cancer are multifaceted and complex. First, many patients demonstrate a high risk of developing thromboembolic disease, known as migratory thrombophlebitis, or Trousseau syndrome,99–103 often seen in patients with mucinous adenocarcinomas or pancreatic and gastrointestinal origin as well as in patients with lung, breast, ovary, and prostate carcinoma. The mechanism is poorly understood but abnormalities of coagulation are seen in up to 92% of these patients.104 Possible causes include increased coagulation factors produced in response to chronic low-grade disseminated intravascular coagulation, circulating cancer-produced procoagulants, tumorf-associated thrombocytosis, and effects of cancer cells exposed to circulating blood.105–107 Hypercoagulability seen in migratory thrombophlebitis in cancer patients is best treated with heparin rather than coumadin.108
Despite the frequent presence of hypercoagulability, patients with cancer have a high incidence of hemorrhagic complications when treated with anticoagulants; the prevalence ranges from 20 to 50%.109–112 Patients with malignancies who are treated with anticoagulation have a significantly higher risk of major complications than those treated with vena cava filter placement,113 which led to the approach of using vena cava filters as the primary therapy in cancer patients with DVT or PE.108,113,114
It has been claimed that vena cava filter placement as primary therapy in patients with cancer and DVT or PE is less expensive than anticoagulation due to the cost of treating hemorrhagic complications during anticoagulation therapy;104 however, the relative benefits of vena cava filter placement depend on expected survival after filter placement. One series reported a 43% in-hospital mortality in patients with advanced cancer and multisystem organ failure who had vena cava filters placed, and benefits of filter placement were considered dubious.115 Others have reported better survival, with in-hospital mortality of 18% in one series116 and 26% in another, which reported that 21 of 61 patients with malignancies who had vena cava filters placed expired within 59 days of filter placement.117 This experience led to the recommendation that patients should be treated while considering the expected longevity and the likelihood of the patient leaving the hospital alive.104,114 In patients who do not have multisystem failure but do have a risk of severe adverse outcome of hemorrhagic complications, such as patients with metastatic disease to the brain or pericardium, filter placement should be the primary treatment of choice for deep venous thromboembolic disease.104 Adjuvant therapy using heparin in patients with tumor-associated hypercoagulability who undergo vena cava filter placement should be considered.117
In contrast to patients with metastatic cancer, most patients admitted for trauma are young and have little or no significant medical history. Therefore, if they survive the initial event, the potential exists for long-term survival and excellent quality of life.
Although fatal pulmonary embolus is rare in trauma patients, occuring in approximately 1% of patients,118,119 PE has been implicated in 11 to 20% of deaths in hospitalized trauma patients.120–122 Prolonged immobility, advanced age, surgical procedures, and extremity and pelvic fractures all contribute to the relatively frequent occurence of DVT in these patients.119–124 The risk of proximal DVT in such patients is approximately 7% with DVT prophylaxis and 18% without DVT prophylaxis.119,123 In these patients, aggressive prophylaxis for pulmonary thromboembolic disease may be warranted.
Prophylactic use of vena cava filters in high-risk trauma patients has been evaluated in two large series.118,122 The overall prevalence of fatal PE in consecutive admitted trauma patients was 0.25%, a significant reduction compared with the 1% rate before the use of prophylactic vena cava filters in high-risk patients.118 The benefits of prophylactic filter placement in high-risk trauma patients was supported in the other large series.122
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree