Figure 22.1 Gastric perforation. Two-year-old abused child. The stepfather admitted to striking the child in the abdomen with his upraised knee. A, Massive free air outlines the peritoneal cavity, the “football” sign. Free air defines the serosal aspect of the small bowel (curved arrows), the falciform ligament (short straight arrow), and the anterior diaphragmatic attachment (long arrow). B, A gross pathologic specimen reveals a large transmural laceration along the proximal greater curvature of the stomach (solid arrows). A large contusion is present along the lesser curvature (open arrows). e, Esophagus. C, A view of the stomach opened along the greater curvature demonstrates several of the 13 mucosal lacerations (arrows). (From Case MES, Nanduri R. Laceration of the stomach by blunt trauma in a child: a case of child abuse. J Forensic Sci. 1983;28:496–501.)
Several older reviews of abdominal injuries in abused children describe only one gastric perforation (9, 19, 20) and cases of inflicted gastric perforation are conspicuously absent from the current literature. The scarcity of reports is similar to that relating to gastric perforation secondary to accidental blunt abdominal injury. However, Jamieson and associates reviewed 43 children with gastrointestinal (GI) perforation over a 10-year period, mostly due to MVAs, and they found 7 gastric perforations (35). There have also been rare reports of gastric rupture after cardiopulmonary resuscitation (CPR) in children (36, 37). Postmortem gastric rupture may occur due to endogenous enzymes, termed gastromalacia, and this may mimic abusive injury (38). Siemens and Fulton indicated that 0.9–1.7% of all hollow viscus ruptures involve the stomach (39). The ruptures generally occur in association with large amounts of gastric contents, and a history of injury after ingestion of a meal is often elicited. A physical struggle may play a role in perforation (40). At laparotomy, the perforations, like those of accidental blunt injury, are usually along the anterior wall or curvatures, and occasionally combined anterior and posterior perforations are noted. The injury may be isolated or associated with other solid and hollow viscus injuries. Gastric rupture is a serious injury that if unrecognized can lead to sepsis, shock, and death (32). Radiologic findings are generally nonspecific, usually with evidence of massive free intraperitoneal air (Fig. 22.1). The dramatic presentation and signs of intra-abdominal sepsis and gross free air generally lead to immediate exploration, precluding any further radiologic studies.
Another very rarely reported gastric injury is an intramural hematoma (41–43). A circumferential blood collection in the antral wall produces luminal narrowing on upper GI (UGI) series (Figs. 22.2A, 22.3A). CT may reveal a subtle diffuse thickening of the wall of the gastric antrum (Fig. 22.2B) or a gross low-density antral intramural blood collection (Fig. 22.3B). The patient reported by Fulcher and colleagues developed gastric pneumatosis, presumed secondary to a gastric mucosal tear (Fig. 22.3C) (42). Another child with suspected abuse had gastric pneumatosis associated with shock bowel and died of his injuries (43). Gastric hematoma may be an isolated injury or may be associated with a small bowel hematoma (see Fig. 22.16). It is probably more common than suggested by the rare descriptions in the literature. A unique case of a giant gastric ulcer in an abused child was reported by Stroh and colleagues (44). This penetrating ulcer of the lesser curvature was noted in a 3½-month-old abused child who presented with hematemesis (Fig. 22.4).

Figure 22.2 Gastric antral hematoma. Four-year-old abused child with a history of vomiting. (See same patient in Fig. 22.16.) A, A spot film from an UGI series shows circumferential narrowing (arrows) of the distal antrum without significant obstruction. B, CT with oral and IV contrast demonstrates circumferential thickening of the antral wall (arrows). The duodenum was normal. This patient also had a large jejunal hematoma.

Figure 22.3 Gastric antral hematoma with pneumatosis. Twenty-one-month-old unresponsive and hypotensive child with multiple bruises, lacerations, and human bite marks. A, An UGI series demonstrates an asymmetric circumferential mass effect upon the gastric antrum (arrows). B, An initial CT scan shows antral hematoma manifested as relative low density surrounding the contrast-filled antrum (arrows). C, Follow-up abdominal CT scan. A cropped view with narrow window setting demonstrates gastric pneumatosis within the fundus (arrow). (From Fulcher AS, Das Narla L, Brewer WH. Gastric hematoma and pneumatosis in child abuse. AJR. 1990;155:1283–4.)

Figure 22.4 Gastric ulcer. UGI series in a 3½-year-old abused child who presented with hematemesis. There is a large, penetrating, lesser curvature giant ulcer (arrow). A follow-up study after three weeks of cimetidine administration showed almost complete healing. (From Stroh A, Zamet P, Bomsel F. [Acute stress ulcer in an abusing family.] Arch Fr Pediatr. 1983;40:411–14.)
A recent report describes a fatal case of Rapunzel syndrome in a neglected, disabled, three-year-old girl. Trichobezoar in the stomach and duodenum led to chronic malabsorption, cachexia, and ultimately death from bronchopneumonia (45).
The small intestine
Perforation
Small intestinal perforation is a well-recognized feature of child abuse and is generally attributed to a blunt blow to the abdomen. The number of reports in the literature undoubtedly fails to reflect the true incidence of this visceral injury. A 1987 literature review (46) revealed only 21 small intestinal perforations occurring in 17 abused children (13, 14, 18, 47–49). The average age of the child was two years, with a range of six months to four years. The distribution of these injuries is depicted in Fig. 22.5. Approximately 60% occurred in the jejunum; when specified, the site was usually just distal to the ligament of Treitz. Thirty percent occurred in the duodenum and 10% in the ileum. Other studies confirm the strong tendency for perforation to involve the duodenum and jejunum (9, 15, 19, 20, 41, 50–56). Wood and colleagues reported that 4 of 13 abdominally injured abused children had duodenal or jejunal perforations, and bowel injuries were significantly more common in abused children than in accidentally injured children in their series (21). Hilmes and associates reported 3 jejunal perforations in 35 children with inflicted abdominal injury (7). In a review of small bowel injuries in children, 7 of 28 cases were due to abuse, and 4 of these children had duodenal or jejunal perforations (57).

Figure 22.5 Distribution of small intestinal perforations in abused children.
Gaines and colleagues reviewed 30 children with duodenal injuries; 8 children were abused and 3 of these had duodenal perforation. All children less than four years of age who suffered a duodenal injury were victims of abuse (58). In several series, accidental duodenal injuries were not recorded in any children less than four years of age (21, 25, 58). Sowrey et al. studied a cohort of patients less than 5 years old admitted with duodenal injuries at 1 of 6 Level I pediatric trauma centers from 1991 to 2011 (59). They identified 32 patients with duodenal injuries with a mean age of 3 years. Duodenal injuries included duodenal hematomas (44%) and perforations/transections (56%). Fifty-three percent of cases underwent operation, 53% had additional injuries, and 12.5% resulted in death. Twenty children (62.5%) were abused and all duodenal injuries in children younger than 2 years (N = 6) were caused by child abuse, and 14 of 26 of the duodenal injuries in children older than 2 years were inflicted. Child abuse-related duodenal injuries were associated with delayed presentation (P = 0.004). There was a significant increase in child abuse-related duodenal injuries during the time frame of the study (P = 0.002). The authors’ evidence supports the practice of a child abuse investigation in children younger than two years with duodenal injury. Studies of duodenal injuries that are predominantly in older children show much lower percentages of cases of abuse (60).
The predilection for the duodenum and proximal jejunum supports the concept that fixation either in a retroperitoneal location, or just distal to the ligament of Treitz, renders the gut susceptible to nonpenetrating injury. This pattern is somewhat at variance with that described in primarily adult clinical series, as well as in experimental studies. Georghegen and Brush reported 20 cases of accidental intestinal perforation and reviewed the literature on the subject (61). They found little relationship between the site of small intestinal perforation and the degree of fixation or length of small bowel mesentery. They performed a series of experiments in which they dropped 50-lb weights from heights varying from 3.5 to 4.5 ft onto the abdomens of intact etherized dogs. They again found no predilection for sites of fixation such as the duodenum or proximal jejunum.
In the abused child, perforations of the duodenum and proximal jejunum occur with direct crushing or compressive forces. Although the resultant sudden increase in intraluminal pressure may cause a perforation, experimental data in dogs suggest that the pressure created is usually insufficient to rupture the bowel (62). It is likely that shearing between apposing surfaces produces most intestinal perforations. The portions of the small bowel suspended by a mesentery may also be injured by a sudden deceleration, typically occurring after a child is thrown or swung into a solid object. This may result in disruption of the vascular supply to the small bowel, with or without perforation (63). Abusive caretakers may report that an injury occurred after a fall on stairs. Huntimer and associates reviewed 312 cases of small bowel perforation and 677 cases of falls on stairs (64). None of the perforations occurred after a fall on stairs and none of the falls on stairs led to significant abdominal injuries.
Free intraperitoneal intestinal perforations are generally associated with impressive findings of abdominal pain, distention, leukocytosis, and fever. Delay in seeking attention is itself an indication of neglect. Retroperitoneal perforations resulting from duodenal injury may be insidious and, although associated with signs of sepsis, patients may not display typical findings of peritonitis. If an uncomplicated intestinal perforation is attended to shortly after the injury, the prognosis is good. Although the patient may be extremely ill, intestinal injury may not be suspected because of a misleading history or neurologic findings that dominate the clinical picture. If other visceral injuries coexist, or if medical attention is delayed, there is a high mortality rate (18).
Imaging features
The radiologic abnormalities related to intestinal perforation depend on the location of the injury. Free intestinal perforations often, but not invariably, result in identifiable free intraperitoneal air. The volume of pneumoperitoneum is usually small and, in contrast to that associated with gastric perforations, is difficult to identify in the supine position. A variety of radiologic signs allow identification of small amounts of free air in the supine position (Figs. 22.6, 22.7).

Figure 22.6 Free intraperitoneal air. Five-year-old abused child. A, Coned-down view of the upper quadrant shows a small collection of air (arrows) between the liver and the anterior abdominal wall. B, Another view reveals a faint collection of free air in the region of Morison’s pouch (open arrows), and combined intraluminal and intraperitoneal air defines the wall of an intestinal loop (solid arrows).

Figure 22.7 Free intraperitoneal air. Four-year-old abused child. A, A supine radiograph of the abdomen demonstrates an ill-defined radiolucency in the upper abdomen (solid arrows) that defines the falciform ligament (open arrows). Upright frontal (B) and lateral (C) views of the chest show free air beneath the dome of both hemidiaphragms (arrows). Jejunal perforation was found at laparotomy.
Collections of free air lying adjacent to gas-filled bowel define the serosal aspect of the gut (Fig. 22.6B). This results in visualization of the inner and outer aspects of the bowel wall, indicating free air – Rigler’s sign (65). This pattern can be simulated by two closely apposed gas-filled bowel loops, but careful analysis of the findings should allow differentiation. Free air may also collect in the subhepatic region (Fig. 22.6B) or between the liver and the anterior abdominal wall, with characteristic patterns (Fig. 22.6A). The free air may outline the falciform ligament (Fig. 22.7A).
Air may collect beneath the anterior diaphragmatic attachments, resulting in a vague radiolucency in the xiphoid region on the supine or semierect chest film (66). Larger collections of air outline the falciform ligament and accumulate centrally beneath the anterior abdominal wall, producing the “football” sign (see Fig. 22.1). An upright view (Fig. 22.7B,C) or, if the patient cannot be placed erect, a cross-table lateral or left lateral decubitus view will confirm the presence of free air. Retroperitoneal perforations are extremely difficult to detect on plain radiographs; however, if sufficient air leaks into the retroperitoneum, or if gas-forming organisms produce retroperitoneal abscesses, a mottled or bubbly pattern will be evident (13).
CT is ideally suited to identify intestinal perforations, particularly those occurring in the retroperitoneal duodenum (see Fig. 22.17) (20, 67–69). The most frequent CT finding associated with bowel perforation is free fluid without evidence of other abdominal injury; free air is only seen in 30–50% of cases (35, 69–71). Free air on CT may be present anterior to the liver, trapped in the leaves of the mesentery or in the retroperitoneum. Other CT findings in the setting of bowel rupture include focal bowel wall disruption, intense bowel wall enhancement, bowel dilatation and wall thickening, and mesenteric stranding or hematoma (Fig. 22.8) (35, 69, 71).

Figure 22.8 Jejunal perforation. Thirty-six-month-old with suspected abuse. A, CT image shows free intraperitoneal air (black arrows). B, C, Additional images show free air (black arrow), small bowel wall thickening (white arrow in B) and free fluid (white arrow in C). A jejunal perforation was found and repaired. (From Trout AT, Strouse PJ, Mohr BA, Khalatbari S, Myles JD. Abdominal and pelvic CT in cases of suspected abuse: can clinical and laboratory findings guide its use? Pediatr Radiol. 2011;41(1):92–8.)
A study of 27 children showed that the presence of retroperitoneal free air or oral contrast reliably differentiated duodenal perforation from contusion/hematoma (72). In another review of duodenal injuries in children, CT showed definite evdience of perforation in only three of five cases of duodenal perforation (73). Imaging features of bowel perforation may also overlap with those seen with the hypoperfusion complex; the latter typically has more diffuse bowel wall thickening, is confined to the small bowel, and is associated with decreased caliber of the inferior vena cava and aorta (see Fig. 22.27) (69, 71).
A normal CT usually excludes the diagnosis of GI perforation (35). However, Canty and associates reviewed a series of injuries to the GI tract in blunt trauma in children and found initial CT negative in 10 children who later were found to have bowel injury (74). Repeat CT will often be diagnostic in the setting of evolving signs of peritoneal irritation. Extraluminal oral contrast is rarely seen in the setting of bowel perforation. Hyperdense free fluid may also be seen with active bleeding, vascular injury, or injury to the urinary tract. In one review of mostly adult patients with suspected blunt duodenal injury, 96 patients with equivocal duodenal injuries on CT went on to have UGI series (75). They found UGI series to have a low sensitivity for the diagnosis of duodenal perforation.
Intramural hematoma
In contrast to intestinal perforations, which are diagnosed by inference from the finding of free intraperitoneal air or fluid, specific radiologically apparent alterations accompany intramural intestinal hematomas that often permit confident diagnosis. Intestinal perforation can have a variety of causes, only one of which is trauma, but in the child without a bleeding tendency the radiologic diagnosis of an intramural hematoma indicates previous trauma.
In 1965, Eisenstein and colleagues described a 38-month-old child with a 6-day history of vomiting (76). A laparotomy one day after admission revealed a gangrenous loop of jejunum containing a large intramural hematoma. Bratu and associates reported the radiologic findings on an UGI series in an abused child with a jejunal hematoma (77). Many reports of small intestinal hematomas have appeared subsequently, and the entity is well accepted as one of the more classic abdominal injuries in cases of child abuse (16, 56, 78–85). Most occur in the second and third parts of the duodenum. Pathologically, hematomas may be diffuse or localized. When well-confined, blood lies mainly in the submucosal and subserosal locations and may be due to direct trauma or ischemic injury (8). In most cases the mucosa is uninvolved, but occasionally ischemic necrosis may occur (86, 87). The mesenteric side of bowel is more prone to vascular tears while the antimesenteric side is more vulnerable to perforation (8). In the jejunum and ileum, bleeding within the small bowel mesentery may be found in association with the characteristic intramural collections.
Anatomic differences between the duodenum and the remainder of the small bowel influence the pathogenesis of the hematomas, as well as their radiologic patterns (Fig. 22.9). Great importance has been placed on the relationship of the transverse portion of the duodenum to the spine. It is postulated that a blunt injury that compresses the duodenum between the anterior abdominal wall and the spine results in duodenal hematoma (56, 62, 84). Because the descending duodenum lies to the right of the spine, additional factors must be considered to explain the conspicuous hematomas frequently encountered in this region. The firm fixation of the descending duodenum in a retroperitoneal location renders the bowel susceptible to a compressive blow, particularly if the child is in a fixed position and is unable to recoil from the assault. The duodenum has a rich vascular supply that is well known to protect it from ischemic injury. The many terminal arborizations of the inferior and superior pancreaticoduodenal arteries are potential sites for extravasation of blood. In the descending duodenum the bleeding is most frequently noted along the lateral margin opposite the site of entry of the nutrient vessels (Fig. 22.10). In the jejunum and ileum the hematomas are usually noted adjacent to the small bowel mesentery, and associated injury and bleeding in the mesentery may be present.

Figure 22.9 Distribution of visceral injuries from blunt abdominal trauma. A midline abdominal blow will compress the abdominal viscera against the spine, resulting in injury to the duodenum (1), left lobe of the liver (2), and pancreas (3). More laterally positioned blows may cause injury to the right lobe of the liver, the kidneys, the adrenal, and occasionally the spleen. Shearing forces generated by a direct blow or sudden deceleration will result in intestinal-mesenteric injuries.

Figure 22.10 Schematic representation of a duodenal hematoma. A, Intact duodenum. B, Coronal section. The hematoma arises in the lateral aspect of the duodenum. The intraluminal mass encroaches upon the lumen from laterally. Contrast material will collect between the mass and the crowded mucosal folds, resulting in the coiled-spring appearance.
The clinical findings in patients with duodenal and jejunal hematomas are characteristic. Vomiting and abdominal pain are almost always present in patients eventually coming to diagnosis. As with other injuries in abused children, it is sometimes difficult to date the specific injury, and in some cases vomiting may be delayed for hours or days after the traumatic insult (56). Blood loss can be significant and there may be elevation of the white blood cell count. If there is associated pancreatic injury, the serum amylase level is usually elevated (56). If there is associated perforation, signs of peritonitis and sepsis develop. In the past, surgical evacuation of intramural hematomas was frequently carried out. It is now generally accepted that a nonsurgical approach should be followed (88), although percutaneous needle aspiration has been rarely performed (89).
Imaging features
The radiographic findings in cases of duodenal hematoma may be normal; however, if significant obstruction is present, a dilated gas- and fluid-filled stomach will be evident, with little intestinal contents distally (Fig. 22.11). On rare occasions, gas within the proximal portion of the duodenum defines the encroaching intraluminal mass (90). Other associated injuries may be manifested by free intraperitoneal air or ascites.

Figure 22.11 Duodenal hematoma. Two-and-a-half-year-old abused child with vomiting, bruises and a palpable abdominal mass. A, An upright view of the abdomen demonstrates a dilated gas- and fluid-filled stomach. B, An UGI series reveals a partially obstructing filling defect in the second portion of the duodenum (arrows). There is the suggestion of a “coiled-spring” appearance. C, The coiled-spring appearance (open arrows) and smoothly marginated hematoma (solid arrows) are seen to advantage in this double-contrast view. D, A follow-up study reveals a residual mural mass in the descending portion of the duodenum (arrows). (From Kleinman PK, Brill P, Winchester P. The resolving duodenal-jejunal hematoma in abused children. Radiology. 1986;160:747–50.)
The characteristic pattern on UGI series is a large intramural mass that encroaches upon the intestinal lumen. The mass is smooth and rounded and may produce varying degrees of obstruction (Fig. 22.11B). With complete obstruction, barium caps the proximal margin of the mass. The appearance in these cases is of a nonspecific obstructing mural process. With less obstruction, varying amounts of barium pass between the mass and the surrounding mucosa, resulting in a “coiled-spring” appearance (Fig. 22.11C). There has been considerable confusion in the literature regarding the use of this term. Some authors have described an intraluminal mass in association with thickened mucosal folds as an example of the coiled-spring appearance (91). A careful reading of Felson and Levin’s classic article on the subject reveals a clear distinction between simple fold thickening related to accumulation of fluid or blood circumferentially within the bowel wall, and an intraluminal mass over which crowded valvulae conniventes are outlined (92). Felson and Levin attempted to simulate the coiled-spring appearance by intramural injection of water but succeeded only in producing thickened folds without the classic radiologic pattern (92). The prerequisite for the production of the coiled-spring appearance is a large intramural mass projecting into the lumen of the bowel (Fig. 22.10). The adjacent mucosal folds are draped over the mass, and there is crowding of the valvulae conniventes; barium trapped within the mucosal folds results in the coiled-spring appearance. A similar appearance is noted in cases of intestinal intussusception, where normal mucosa of the intussuscipiens is draped over the intussusceptum, producing the coiled-spring effect. Thus, although some degree of fold thickening may be present, it is the intraluminal mass that is the essential component for the production of the coiled-spring sign (93). This sign is not specific for duodenal hematomas; it may be even more impressive in the jejunum (Fig. 22.12, also see Fig. 22.16).

Figure 22.12 Duodenal and jejunal hematomas. A 2½-year-old abused infant with multiple bruises and no history of trauma. A, UGI series shows barium trapped between the intramural mass and the medial and superior margins of the second and third portions of the duodenum (white arrows). Barium also defines the margins of an intramural jejunal hematoma with a coiled-spring appearance (black arrow). B, Abdominal ultrasonography reveals a large, anechoic, tubular structure consistent with a jejunal hematoma (arrows). (From Radkowski MA, Merten DF, Leonidas JC. The abused child: criteria for the radiologic diagnosis. Radiographics. 1983;3:262–97.)
In the descending duodenum, the intramural mass characteristically projects from the lateral margin or greater curvature aspect of the duodenum (Fig. 22.12), but occasionally the mass appears to arise medially (94). This feature is extremely helpful in evaluating cases of resolving hematomas, because the lateral location of the mural defect will remain constant (Figs. 22.11, 22.13). As the mural mass extends into the third portion of the duodenum, it arises from the inferior margin of the bowel and is outlined superiorly by intraluminal barium (Fig. 22.12) (95). While some authors have found that duodenography is not useful for differentiating duodenal hematoma from perforation in adults (75), in another review, duodenography identified duodenal hematomas in four children not seen on initial CT scans (73). Although CT has become the mainstay for detection of bowel wall injury, the UGI is by no means obsolete and it should be selectively employed in the initial evaluation of suspected duodenal hematoma.
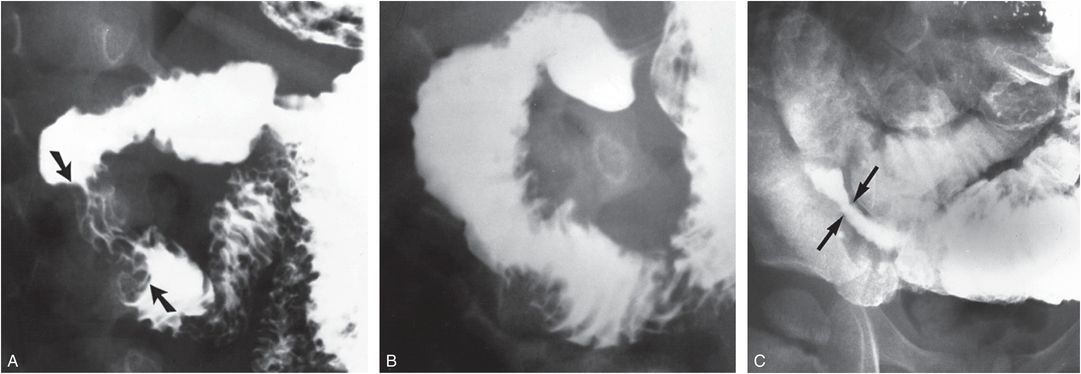
Figure 22.13 Resolving duodenal hematoma. Three-year-old child with abdominal pain and distention, vomiting, and guaiac-positive stools. Chylous ascites was diagnosed by paracentesis. A, UGI series shows a lobulated mural defects arising from the lateral aspect of the descending duodenum (arrows) in association with thickening of the mucosal folds. B, Follow-up study three weeks later shows a decrease in the size of the mural defects. C, Delayed coned-down view of the right lower quadrant demonstrates a stenosis in the distal small bowel (arrows) with proximal dilatation. Five months later the child was found murdered at home with multiple visceral and central nervous system injuries. (From Kleinman PK, Brill P, Winchester P. The resolving duodenal-jejunal hematoma in abused children. Radiology. 1986;160:747–50.)
Sonography and CT are routinely used in the assessment of intramural duodenal hematomas. Both modalities can provide documentation of the initial injury, and CT is indispensable for defining complications associated with both duodenal and more distal small bowel perforations (54, 89, 96). The sonographic appearance depends on the location, extent, and age of the hematoma. If the intramural blood is localized to the second portion of the duodenum, it is evident as a relatively well-defined mass lying anterior to the right kidney and inferior vena cava. More extensive hematomas involving the transverse portion of the duodenum extend across the midline anterior to the aorta (Fig. 22.14A). Acutely, there is increased homogeneous echogenicity throughout the mass. Gradually a pattern of mixed echogenicity evolves and ultimately an anechoic cystic mass becomes evident. The nature of the lesion is confirmed with an UGI that shows the mass encroaching upon the bowel lumen (Fig. 22.14B) and CT fully delineates the extent of the mass and its relationships to surrounding structures (Fig. 22.14C) (96). Although sonography is a rapid and often useful diagnostic imaging tool, it may be difficult to obtain adequate images when a large amount of intestinal gas is present. CT provides more elegant delineation of the intramural hematoma and its complications. These studies are best performed with oral contrast, but rapid multi-detector CT (MDCT) scanners may provide diagnosis without intraluminal contrast (69). As with sonography, features depend on the extent and the age of the injury. Intramural blood may be manifested as a large discrete mass or may produce only modest bowel wall thickening (Fig. 22.15). Typically, there is focal, eccentric bowel wall thickening which may be associated with proximal obstruction (70). Thickening of the duodenal wall and fluid collections in the anterior pararenal space may be seen with both perforation and bowel wall contusion (97). A study of 27 children showed that retroperitoneal free air or oral contrast reliably differentiated duodenal perforation from contusion (72). Usually duodenal hematomas have mixed attenuation (98). Initially, intramural blood may be of relatively high attenuation. With time, attenuation diminishes, approaching that of water. CT acquired in the decubitus position may be particularly useful for delineating these intramural blood collections (Fig. 22.15B).

Figure 22.14 Duodenal hematoma. A 21-month-old child with vomiting and elevated transaminases. A, Transverse abdominal US shows a complex, rounded mass in the upper mid abdomen (arrows). B, UGI shows eccentric luminal narrowing in the third/fourth portion of the duodenum (arrows). C, Abdominal CT shows a hypodense, sausage-shaped structure in the expected location of the duodenum (arrowheads).

Figure 22.15 Duodenal hematoma and other abdominal injuries. Two-and-a-half-year-old child with a history of vomiting that had resolved. An isolated fracture of a right lower rib (not shown) prompted a determination of serum liver transaminase levels, which were elevated. A, An abdominal CT scan with oral and IV contrast demonstrates an extensive intramural hematoma that projects into the lumen of the second portion of the duodenum (large arrow) and circumferentially narrows the third portion (small arrows). B, An image in the right lateral decubitus position shows the intramural blood to advantage (arrows). C, D, Although CT revealed no gross hepatic parenchymal injury, pericholecystic fluid is noted by CT (arrows in C) and also by sonography (arrows in D). G, gallbladder; L, liver. E, A moderate-sized low-density adrenal hemorrhage (arrow) is also evident on CT.
Duodenal hematomas are frequently continuous with proximal jejunal injuries and thus can straddle the ligament of Treitz (see Fig. 22.12). Jejunal hematomas may also be isolated intestinal injuries and can present particular diagnostic problems when encountered in a chronic phase. Figure 22.16 illustrates the case of a four-year-old girl who presented with vague abdominal complaints and vomiting. There was no evidence of trauma. Abdominal sonography demonstrated a large, anechoic, sausage-shaped mass in the left abdomen. Strandy areas of echogenicity suggested a resolving hematoma. CT revealed a low attenuation mass extending from the left upper quadrant to below the lower pole of the left kidney. In addition to intramural hematoma, the differential diagnosis included other cystic abdominal masses. Subsequent UGI series with small bowel follow-through showed the classic coiled-spring appearance of a jejunal hematoma. Skeletal imaging studies revealed healing fractures. After many initial denials, the child eventually indicated that she had been kicked in the abdomen by her mother’s boyfriend several weeks earlier.

Figure 22.16 Jejunal hematoma. Four-year-old child with previous vomiting. (See same patient in Fig. 22.2.) Sagittal (A) and transverse (B) sonographic images show a primarily cystic, sausage-shaped mass (M) in the left abdomen. The mass lies anterior to the abdominal aorta (A) in the sagittal image. In the transverse view, echogenic material (arrows), presumably settled blood products, is noted within the dependent portion of the mass. C, CT shows a low attenuation mass (M) anterior to the left kidney. D, A small bowel series with barium demonstrates a large intramural jejunal mass (arrows) beginning just distal to the ligament of Treitz. E, A coned-down view shows a coiled-spring appearance and a smooth distal margin of the intramural mass (arrow). A gastric antral hematoma was also present.
Although scintigraphy is not generally used in this context, there are several reports of inflicted intramural hematomas detected on radionuclide imaging. Mitchell and colleagues described a 10-month-old abused child with duodenal hematoma evaluated with scintigraphy (89). They documented accumulation of 99mTc-labeled red blood cells (RBCs) in the region of the duodenum that persisted on delayed images. The duodenal hematoma was subsequently documented with sonography and percutaneously aspirated (89). In another reported case, a delayed image from a bone scan showed tracer accumulation in the upper mid-abdomen associated with a duodenal hematoma in an abused two-year-old child (99).
Small intestinal strictures
Small bowel strictures are well-recognized complications of accidental blunt abdominal trauma, particularly after lap-belt injury (100, 101), and small bowel strictures have rarely been described in association with abuse (46, 51, 54). Intramural hematomas, particularly when associated with a mesenteric injury, may lead to fibrosis and narrowing of the intestinal lumen. Small bowel strictures may also occur when trauma results in devitalization of a small bowel segment with localized perforation. Shah and colleagues described a jejunal stricture complicating inflicted duodenal hematoma and contained perforations of the duodenum and proximal jejunum in a 15-month-old abused boy (Fig. 22.17) (54). Initial CT demonstrated a duodenal hematoma, and an UGI series showed a contained distal duodenal perforation. Subsequent UGI series demonstrated high-grade obstruction in the distal duodenum, and exploratory laparotomy revealed strictures of the distal duodenum and jejunum. Multiple osseous injuries were noted on skeletal survey (SS), including a thoracic spine fracture. Because mesenteric injuries and intramural hematomas can occur throughout the small bowel, stricture formation may be evident in the distal small intestine with associated partial small bowel obstruction (see Fig. 22.13C).

Figure 22.17 Duodenal jejunal hematoma: perforation and stricture formation. Fifteen-month-old boy with vomiting. A, CT of the upper abdomen shows a low-density intramural hematoma (large straight arrow) in the second portion of the duodenum. Wall thickening (small straight arrows) is seen in the third portion, possibly associated with extraluminal gas (curved arrow). B, An UGI series several days later demonstrates marked deformity of the duodenum and a featureless mucosal pattern with a fixed focal collection of contrast consistent with contained perforation (arrow). There is marked dilation of the distal duodenum and jejunum. C, Lateral spot film from an UGI series four weeks later shows high-grade duodenal obstruction (arrow). (From Shah P, Applegate KE, Buonomo C. Stricture of the duodenum and jejunum in an abused child. Pediatr Radiol. 1997;27:281–3.)
Miscellaneous lesions
Hematomas can be present within the mesentery without bowel involvement and are often associated with disruption of large mesenteric arteries. This may then lead to bowel ischemia or infarction. Mesenteric hematomas can be delineated by CT with both intravenous (IV) and oral contrast (Fig. 22.18). Pneumatosis intestinalis and portal venous gas are common in premature infants with necrotizing enterocolitis and may occur in abused children (Fig. 22.19) (1, 5, 82, 102). A variety of mechanisms may explain this pattern, including a rent in the mucosa with entry of gas into the bowel wall or an ischemic injury resulting in intramural air due to gas-forming organisms. The intramural gas appears as curvilinear, mottled, and occasionally cystic lucencies related to bowel loops. Once air has entered the bowel wall, it may pass to the portal venous system with a characteristic radiographic pattern of branching radiolucencies in the hepatic region.

Figure 22.18 Mesenteric hematoma. A 13-month-old abused child with an initial reported history of a fall from a couch. CT of the abdomen shows a heterogeneous mesenteric hypodensity consistent with a hematoma (H). There were also liver and renal contusions, skull fracture, SDH and bilateral retinal hemorrhages. (From Strouse PJ, Close BJ, Marshall KW, Cywes R. CT of bowel and mesenteric trauma in children. Radiographics. 1999;19(5):1237–50.)

Figure 22.19 Pneumatosis intestinalis and colonic hematoma. A two-year-old abused child. A, Supine radiograph of the abdomen shows pneumatosis intestinalis (white arrow) and portal venous gas (black arrows). B, A barium enema reveals a circumferential intramural mass involving the ascending colon (arrows). At laparotomy a 6-cm hepatic flexure hematoma was found associated with a 5-cm duodenal hematoma. Liver laceration and pneumatosis intestinalis were also present without gross intestinal perforation. (From Mueller GP, Cassady CI, Dietrich RB, Pais MJ, Warden MJ. Pediatric case of the day. Occult child abuse (manifesting with pneumatosis intestinalis and portal venous gas). Radiographics. 1994;14:928–30.)
Intussusception is a rare manifestation of abuse. In a review of 24 cases of intussusception, Walker and Giltman encountered a battered child in whom an intussusception was unexpectedly discovered during laparotomy (103). Intramural hematomas are well known to result in intussusception in patients with bleeding disorders and Henoch–Schönlein purpura, and this is the presumed mechanism when intussusception is associated with abuse. Transient small bowel intussusceptions are familiar incidental findings on abdominal CT and in most instances, are presumably normal phenomena. When multiple intussusceptions are encountered in the context of other visceral and skeletal injuries, when the small bowel is otherwise normal, their significance is problematic (Fig. 22.20).

Figure 22.20 Multiple small bowel intussusceptions. An eight-month-old infant with facial and abdominal bruises, bite marks, elevated liver enzymes, and a history of falling off the bed. (See same patient in Fig. 21.43.) A, B, Coronal CT reformats of the abdomen demonstrate multiple intraluminal defects (thin arrows) within the contrast-filled small bowel consistent with intussusceptions. There is no small bowel mural thickening to suggest intramural hematoma. These findings are indeterminate. Abuse was supported based on a left lobe liver laceration (thick arrow in B) and multiple spinal fractures.
The colon
Colonic injuries due to blunt trauma are rare in abused children. Intramural hematomas have been described in the ascending, transverse, and descending colon and rectum (16, 18, 50, 51, 102, 104, 105). The hematomas have been diagnosed with barium enema, endoscopy, and CT. On barium enema, asymmetric circumferential narrowing of the bowel lumen is noted (see Fig. 22.19). Because contrast enemas are not commonly performed in cases of suspected abuse, and intramural hematomas tend to resolve with time, it is likely that these injuries are more common than the literature suggests. Sheybani et al. illustrated the case of an abused 17-month-old infant who developed descending colonic pneumatosis followed several weeks later by a corresponding colonic stricture (5). Gornall and associates described a 2½-year-old abused/neglected child with a colonic perforation found at surgery (16). The child presented with severe peritonitis and died two days after laparotomy. Contostavlos and colleagues reported seromuscular rupture of the colon without acute perforation in a two-year-old abused child (50). In another review of 13 children with inflicted abdominal injuries; one had colonic contusions and another had ischemic colon along with multiple other injuries (21).
Distal colonic and anal injuries are more common with sexual abuse but are rarely documented radiographically. Press and colleagues described a 4-year-old boy who presented with 15 cm of small bowel protruding from the rectum (106). At laparotomy the small bowel had eviscerated through a tear in the rectosigmoid. Although abuse was presumed, the mechanism of injury was obscure. Another recent report described forced insertion of a carrot into the rectum of a two-month-old child, leading to distal bowel obstruction (see Fig. 23.28) (107). The colon may be involved in other forms of maltreatment, including impalement with needles and Munchausen syndrome by proxy (see Chapter 23).
The pancreas
Pancreatitis and pseudocysts
For many years the pancreas eluded accurate assessment by standard radiologic techniques. Acute pancreatic injury and its complications were generally defined at surgery or postmortem examination. Although barium contrast studies provided secondary evidence of pancreatic inflammation or mass, manifested as deformity or displacement of contrast-filled bowel, noninvasive direct visualization of the pancreas was impossible. With the advent of sonography, CT, and magnetic resonance imaging (MRI), disturbances in the gross structure of the pancreas are now easily and accurately definable. These techniques have greatly enhanced our ability to detect pancreatic pseudocysts in abused children, and the capacity to monitor this condition noninvasively has had a significant impact on medical and surgical management.
In contrast to adults, in whom up to 80% of pancreatic pseudocysts are due to complications of alcoholic pancreatitis (108), most pseudocysts in children are due to blunt abdominal injury. Cooney and Crosfeld, reporting on 15 children with pancreatic pseudocysts and reviewing additional cases from the literature, found that 60% were due to trauma (109). In 32% the cause was unknown; undoubtedly, some of these were secondary to unrecognized blunt abdominal trauma. Ziegler and colleagues found that 16 of 49 cases (33%) of pancreatitis in children were due to trauma and that 5 cases (10%) were due to abuse (110).
In 1964, Kilman and associates described a child with a large pancreatic pseudocyst, with incidental fractures of three ribs and a clavicle (111). The authors felt that this was perhaps a case of child abuse. In 1967, Kim and Jenkins described a three-year-old child with a large pseudocyst in the retrogastric region (112). Although there was evidence of bruises and scars, and investigations supported abuse, legal proof of physical abuse was not obtained. On the insistence of the mother, the child was discharged to her care. Four months after discharge the child was brought to another hospital dispensary, dead on arrival. The child had suffered a recent fractured arm, a skull fracture, acute subdural hematoma (SDH), and multiple contusions. Waseem and colleagues described a two-year-old child with vomiting, abdominal pain, and poor feeding who presented with no history of trauma (113). She had minimal bruising and abdominal tenderness, hypoactive bowel sounds, elevated liver transaminases and amylase. CT confirmed pancreatic laceration due to confirmed abuse.
It is estimated that up to one-third of cases of traumatic pancreatitis in children are inflicted (114). In a recent review of 35 abdominally injured abused children, three had pancreatic transections and one had acute necrotizing pancreatitis; all required surgical management, and all pancreatic injuries were associated with other injuries (7). Trokel and colleagues reviewed cases of blunt injury in children and found that pancreatic injury was significantly associated with abuse (10). Roaten and associates found 4 of 24 abdominal organ injuries in a group of abused children involved the pancreas and, in another review, 8 of 13 children with inflicted abdominal injuries had pancreatic involvement (21, 22). Likewise, inflicted pancreatic injury is associated with a significantly higher risk of an associated hollow organ injury (115). Numerous additional reports of pancreatitis and pseudocysts associated with abuse have appeared, and the entity is now sufficiently characteristic for diagnosis to have become routine (1, 2, 4, 51, 66, 80, 104, 116–129).
Three critical ingredients are required for the development of pancreatitis: (1) disruption of acinar or ductal integrity; (2) seepage of pancreatic enzymes into tissue spaces; and (3) activation of proteolytic and lipolytic enzymes (130). The pancreas lies in a precarious position, extending from the medial aspect of the descending duodenum upward across the spine and ending in the region of the splenic hilum. The pancreatic body is particularly vulnerable to blunt abdominal trauma, and impact to any region may result in acinar and ductal injury. Enzymatic autodigestion of the organ and hemorrhage result in a pathologic pattern of hemorrhagic pancreatitis. As the process continues, fluid collections containing pancreatic enzymes develop in and around the organ. Initially, these collections may be loosely confined, but with time a firm fibrous capsule develops. Diffuse fluid collections are generally referred to as effusions, and the more discretely confined collections as pancreatic pseudocysts. Secondary infection of the pancreatic and mesenteric collections also occurs (110, 131, 132).
Development of pancreatitis may be rapid, occurring within hours of the injury, or may be gradual and insidious. Although symptoms may be modest immediately after the injury, a factor that may result in failure to seek attention, a consistent pattern of complaints and physical findings can be expected. Vomiting, abdominal distention, and fever develop in most patients. Serum amylase levels are usually elevated and may return to normal despite continued illness (133). Amylase may remain normal in 40% of cases up to 2 days after injury (98). Several authors have found that specificity and sensitivity of initial serum amylase in post-traumatic pancreatitis is low and serum amylase does not correlate with degree of pancreatic injury (134–136). Ascites is often detectable, and paracentesis may yield fluid with an elevated amylase level. Pleural effusions, frequent in adults with pancreatic disease, are uncommon in abused children with pseudocysts. Bruises, scars, and burns may be noted upon inspection. Skeletal surveys may reveal fractures (51, 66, 111, 116, 117, 119, 120, 124, 125, 128). However, there may be no physical evidence whatsoever to suggest abuse, and the cause of the process may go undiscovered until additional injury occurs (4, 66).
Imaging features of pancreatitis
The abdominal radiograph may provide a clue to the presence of pancreatitis. Several radiographic signs have been described with pancreatic inflammatory disease, but these can be noted in other conditions (137). Localized dilation of bowel loops with air–fluid levels, or “sentinel loops,” in the region of the pancreas may be noted with acute pancreatitis. An abrupt termination of a gas-filled proximal transverse colon, or “colon cut-off” sign, is another nonspecific feature of pancreatic inflammation. A mass effect on the descending duodenum and separation of the stomach from the transverse colon are additional findings that may be evident if sufficient intraluminal gas is present.
In acute pancreatitis, sonographic studies may be normal or reveal increased size and decreased echogenicity of the gland (110, 133). MDCT is the imaging study of choice for suspected pancreatic injury. It may show pancreatic enlargement with heterogeneous low density, reflecting intraparenchymal fluid (5). Extrapancreatic effusions may be evident in the perihepatic and subphrenic spaces, as well as in the lesser sac and pelvis. Unexplained fluid in the anterior pararenal space or lesser sac are often the only finding in pancreatic injury in children (70). Fluid may also dissect between the pancreas and splenic vein, though this is not a specific sign of pancreatic injury (138). Stranding of peripancreatic fat and thickening of the anterior renal fascia may also be present.
Although most pancreatic injuries result in diffuse parenchymal insult without gross disruption of parenchyma, actual pancreatic transections and pseudocysts occur and are well documented by CT and sonography (Fig. 22.21; also see Figs. 22.24, 22.28) (1, 5, 7, 20, 120, 127). Pancreatic laceration or transection appears as a linear area of decreased attenuation on CT, though sometimes CT fails to detect this, especially in the acute setting (139). Identification of pancreatic ductal injury may indicate a need for surgery, though nonoperative management is also successful in some instances (129, 140). MRI has also been advocated for blunt pancreatic injury (141), and endoscopic retrograde pancreatography and MR cholangio-pancreatography may allow further delineation of the pancreatic duct for surgical planning (5, 142). Chronic pancreatitis is an occasionally reported consequence of abuse. Chen and associates described the sonographic and CT findings of chronic pancreatitis complicated by acute duodenal obstruction in a seven-year-old abused child (118).

Figure 22.21 Pancreatic transection and pseudocyst with massive chylous ascites. A, Abdominal CT scan demonstrates a transection of the pancreatic neck (white arrow) with associated pseudocyst (P). The pancreatic duct is prominent (black arrows). Extensive chylous ascites (A) is present. B, Sonography demonstrates the pseudocyst (P) extending between the margins of the transected pancreas (arrow). The patient recovered without surgical intervention. (From Hilfer CL, Holgersen LO. Massive chylous ascites and transected pancreas secondary to child abuse: successful non-surgical management. Pediatr Radiol. 1995;25:117–19.)
The process may extend into the transverse mesocolon with elevation of the stomach and displacement of the transverse colon (116). Spiculation of the mucosa or mass effect upon the medial aspect of the descending duodenum provides additional evidence of pancreatic inflammatory disease. Complete duodenal obstruction may occur with pseudocysts in the head of the pancreas (125). Thickening of the folds in the duodenum and jejunum may reflect associated intramural bleeding, although dissection of pancreatic enzymes to these regions can produce a similar appearance. It is not surprising that duodenal hematomas and traumatic pancreatitis can coexist (18) and they may be encountered together at autopsy (143).
Imaging features of pancreatic pseudocysts
Pseudocysts are occasional sequelae of inflicted pancreatic injury, and since they can be relatively silent clinically, patients may be imaged sonographically for modest nonspecific abdominal complaints. Sonography has not only provided enhanced identification of pseudocysts, but further elucidated the natural history of the process (Fig. 22.21). CT is well suited to evaluation of pancreatic pseudocysts (Figs. 22.21, 22.22) (1, 79, 80, 129). It can delineate their location, size, and extent. It also provides optimal assessment of peripancreatic and remote intra-abdominal fluid collections. Experience supports a conservative approach in most cases, except when there is clinical deterioration or persistent elevation of serum amylase levels. An enlarging cyst per se is not a specific indication for surgery and percutaneous drainage of enlarging pseudocysts is generally the preferred approach in the presence of persistent clinical and laboratory signs of activity. Whether catheter drainage or surgery is employed, sonography is preferred to monitor the size of the cyst, but CT will be required for a more detailed assessment when the response to therapy is suboptimal.

Figure 22.22 Pancreatic pseudocyst with anterior and posterior rib fractures. A three-year-old boy with bruises, burns, “cauliflower” ears, and absent front teeth. A, CT of the upper abdomen shows a large hypodense mass in the mid abdomen, consistent with pancreatic pseudocyst (p). B, CCJ (curved arrow) and posteromedial rib fractures (arrow) were also present, as well as skull and humeral fractures (not shown). (From Ng CS, Hall CM. Costochondral junction fractures and intra-abdominal trauma in non-accidental injury (child abuse). Pediatr Radiol. 1998;28(9):671–6.)
Osseous changes due to pancreatitis
Systemic release of enzymes after blunt injury to the pancreas may result in fat necrosis at distant sites (144–146). Medullary fat necrosis may be associated with the production of lytic osseous lesions (145, 147–149). Several reports described similar osteolytic lesions in children with pancreatitis secondary to abuse (104, 121, 123, 125). Bone lesions have been noted as early as 2 weeks and as late as 10 weeks after the onset of abdominal symptoms. The symptoms usually involve the lower extremities, typically the small bones of the feet. Soft tissue swelling and tenderness may be noted over the dorsum of the feet.
The radiologic appearance is of multiple lytic areas of varying size (Fig. 22.23). The number of lesions may be in excess of 300 (121). The patterns range from a “moth-eaten” appearance to punched-out geographic areas of destruction. With healing, increasing sclerosis is noted and periosteal new bone is incorporated into the cortex. The lesions have a predilection for the small bones of the feet, as well as for the metaphyseal regions of the long bones. Epiphyseal involvement is unusual but has been described (104). These lesions appear to develop after the acute phase of the pancreatic inflammatory process, and resolution is spontaneous regardless of treatment. Radiographic changes persist long after clinical findings have resolved, and mild residual changes may be evident as late as one year after diagnosis (122).

Figure 22.23 Osteolytic lesions secondary to pancreatitis. Three-year-old abused child with abdominal tenderness and elevated amylase levels. An initial SS showed only a healing fractured rib. Views of the lower legs (A) and forearm (B) reveal permeative and “moth-eaten” areas of destruction throughout the bony structures. SPNBF (arrows) is noted at multiple sites. Several punched-out geographic areas of destruction are seen (arrowheads). The lesions resolved in six months, but there was persistent increase in overall bony density. (From Neuer FS, Roberts FF, McCarthy V. Osteolytic lesions following traumatic pancreatitis. Am J Dis Child. 1997;131:738–40.)
These destructive changes bear considerable similarity to the osseous lesions associated with disseminated malignancy. The finding of lytic destructive lesions with associated subperiosteal new bone formation (SPNBF) often raises the possibility of leukemia and metastatic neuroblastoma. As the patient may present with systemic illness accompanying abdominal complaints, care should be taken to avoid confusing these entities. In the child presenting with multiple painful lytic lesions involving the small bones of the extremities, serum amylase determination is prudent.
The liver
Hepatic injuries are familiar injuries in abused children and are generally presumed to be due to direct blows to the abdomen or to an impact after sudden deceleration (see Fig. 22.9) (1, 4, 5, 7, 9, 12–14, 16, 17, 19, 20, 26, 51, 80, 83, 117, 121, 123, 143, 150–152). Rarely, penetrating injuries resulting from needles inserted into the abdomen may be encountered unexpectedly on radiographic examination (see Chapter 23) (153–156).
In the early literature on child abuse, hepatic injuries were considered quite rare. Injuries were generally found at laparotomy or autopsy. But with newer imaging technologies, the importance of hepatic injuries has emerged. The liver was injured in 47% of children with inflicted abdominal injuries described by Ledbetter and colleagues (19). The liver was second only to the hollow viscera (65%) of children as the site of visceral injury. Cooper and associates found that when hepatic injury was associated with profound shock due to massive hemorrhage and/or hepatocaval or cavamesenteric disruption, the mortality rate was 83% (9).
In another review of 13 children with inflicted abdominal injury, 12 of 13 had hepatic injury, incuding post-traumatic hepatitis, contusion, and laceration (21). In the review by Hilmes and associates there were 15 liver injuries in 35 abused children with abdominal injuries; the liver was the most commonly injured solid organ (7). In another report of 20 children with inflicted abdominal injury, 11 had hepatic injury ranging from hematoma to rupture (25). Roaten and colleagues reported 8 liver injuries in 24 abused children with abdominal trauma (22).
A study by Coant and associates in 1992 suggested that hepatic injuries are more common than generally reported in abuse and the mortality rate is substantially lower than indicated in the literature (26). These authors used serum transaminase and lactate dehydrogenase (LDH) levels to detect occult liver injury in cases of physical abuse. They studied 49 children who were suspected of being abused but showed no clinical signs of abdominal injury. The transaminase and LDH levels were elevated in 4 cases, and 3 of these (6%) were found by CT to have hepatic lacerations. Two of these patients were under six months of age, a distinctly unusual age group for inflicted hepatic injuries. All patients survived.
More recent reports have confirmed that elevated transaminases can be used as a marker for abdominal injury in abuse. Lindberg and colleagues did a prospective multicenter study to determine the sensitivity and specificity of transaminase screening in abused children (157), and a follow-up retrospective study in 2013 (3). They concluded that imaging of the abdomen in suspected abuse should be performed when the aspartate aminotransferase (AST) or alanine aminotransferase (ALT) values are greater than 80 IU/L, or when the physical examination points towards abdominal injury. Other reviews support screening with transaminases after blunt abdominal injury in children and recommend a variety of cut-off values of transaminases before imaging, ranging from 70 to 450 IU/L (158–162). Trout and associates found that AST and ALT values greater than twice normal were significantly associated with CT evidence of abdominal injury in abused children (43). In another study, all children with inflicted liver injuries who had transaminases drawn had elevated levels (7). Karam and colleagues noted that while patients with liver injury had increased transaminases, several patients with severe liver injury had levels lower than the cut-off values reported in the literature (163). They concluded that low liver function tests (LFTs) cannot rule out liver injury. Likewise, Capraro and associates found that no laboratory test had adequate sensitivity to screen for abdominal trauma in children (164).
Although small hepatic contusions, lacerations, and hematomas may be of little immediate clinical significance, documentation of these injuries may have important forensic implications. Baxter and colleagues have suggested that transaminases can be used to establish time of hepatic injury and, therefore, detect subacute injuries that may occur with inflicted injury (165). Hepatic transaminases rise rapidly after liver injury and fall predictably; when ALT is greater than AST, this is specific for injury greater than 12 hours. These values could be used to date hepatic injuries in suspected abuse.
A report by Dorandeu and associates described histologic evidence in four cases of fatal inflicted abdominal trauma; they found numerous hemosiderin-laden macrophages and other signs of tissue repair in the injured organs, including the liver, suggesting that the acutely injured areas had potentially sustained prior trauma (166). Histologic documention of older abdominal injuries could be used to support a diagnosis of chronic physical abuse. Dedouit and colleagues showed the value of postmortem abdominal CT in a fatally abused child without a history of trauma (143). CT showed liver laceration, healing rib fractures, hemoperitoneum, and pneumoperitoneum, later confirmed at autopsy.
Imaging features
Although the abdominal radiograph may provide some indication of hepatic injury when there is a large amount of intra-abdominal blood, and sonography can show gross parenchymal injury and subcapsular bleeding, CT is currently the definitive diagnostic imaging study. Experience with MRI is limited in this context.
Hepatic injuries seem to be more common in the midline portions of the organ (i.e., the left and caudate lobes) in contrast to right lobe involvement with accidental injury. The left lobe is more vulnerable as it is compressed over the spine with a blow to the abdomen (Figs. 22.24–22.26; also see Figs. 22.9, 22.20) (16, 20). Hepatic injuries include linear lacerations or fractures, hepatic contusions, hematomas, and vascular injuries. On ultrasonography (US), liver hematomas are hyperechoic in the acute phase; within days they become hypoechoic or anechoic, and they may eventually calcify. On CT, liver lacerations appear as linear or irregular areas of low attenuation, often associated with hematomas. Vascular hepatic injuries may appear as wedge-shaped areas of the liver that do not enhance. Blood may dissect along the intrahepatic portal radicals, producing so-called periportal tracking (Figs. 22.25–22.27).

Figure 22.24 Multiple abdominal injuries shown by CT. A sixteen-month-old child with vomiting and abdominal distention. A, Hepatic injuries involve both medial and lateral segments of the left lobe and the caudate lobe of the liver (solid arrows). There is periportal tracking. Low-density adrenal hemorrhage (open arrow) is also noted, separating the two limbs of the gland. B, Areas of decreased perfusion (arrows) are noted in the left kidney consistent with contusion. (From Nimkin K, Teeger S, Wallach MT, DuVally JC, Spevak MR, Kleinman PK. Adrenal hemorrhage in abused children: imaging and postmortem findings. AJR. 1994;162:661–3.)

Figure 22.25 Liver laceration. A five-month-old infant with head and skeletal injuries. (See same patient in Figs. 19.24, 21.19.) A, CT shows both a hypodense liver laceration in left hepatic lobe (black arrow) as well as an enhancing left lobe mass (arrowhead) consistent with a hemangioma. Note CCJ rib fractures (white arrow). B, Coronal reformat shows the laceration (thick arrow) and periportal tracking (thin black arrow). Note CCJ fractures (white arrows) C, Coronal STIR MR images shows hyperintense laceration (thick arrow) and healing rib fractures (thin arrows). (From Perez-Rossello JM, Connolly SA, Newton AW, Zou KH, Kleinman PK. Whole-body MRI in suspected infant abuse. AJR. 2010;195:744–50.)

Figure 22.26 Grade IV left hepatic lobe laceration with fracture of left twelfth rib. Seven-year-old who developed abdominal pain after reportedly falling down a flight of stairs. A, CT shows large hypodense left liver laceration (arrows). B, Coronal oblique reformatted CT image shows left posteromedial twelfth rib fracture (arrow). She subsequently indicated that an adult had stepped on her abdomen.

Figure 22.27 Multiple abdominal injuries and hypoperfusion syndrome. A, CT in a 19-month-old abused comatose child shows a fracture (large arrow) through the caudate lobe of the liver, periportal and retrocaval blood (small arrows), and absent perfusion of the spleen (S). B, CT at a lower level shows pancreatic transection (curved arrow) and intraperitoneal fluid. The inferior vena cava is collapsed (solid arrow). The nephrograms are dense, but there is no contrast in the collecting systems. C, A more inferior section shows a collapsed IVC and intense enhancement of dilated bowel loops. Intracranial injuries were present and the child died 30 minutes after the CT scan was performed. (Courtesy of Donald Kirks MD.)
Periportal tracking may be the only finding evident on CT, but this is nonspecific and not necessarily indicative of periportal blood. Blood may accumulate around the inferior vena cava and in the perihepatic regions and may be associated with a large amount of intraperitoneal blood. Hemoperitoneum is present in approximately two-thirds of cases of hepatic injury (70). Although the most extensive hepatic injuries are more likely to be associated with severe hemorrhage, decisions regarding operative management are generally based on the physiologic status and other associated organ injuries, such as the spleen, pancreas, and kidneys (Figs. 22.24, 22.27) as well as hollow viscera. Major associated vascular injuries, including those to the hepatic veins and inferior vena cava, all have a strong impact upon physiologic status and patient outcome (9). Pericholecystic fluid may be noted in the absence of other imaging features of hepatic injury. In the patient with elevated liver enzymes, this probably reflects blood associated with occult parenchymal disruption. Sonographically, the fluid presents a halo of low echogenicity around the gallbladder margin; on CT it shows an attenuation intermediate between gallbladder and enhanced hepatic parenchyma (see Fig. 22.15). As noted above, the association of lower rib fracture and liver lacerations is a recurrent theme in the child abuse literature (Figs. 22.25, 22.26; also see Fig. 22.34). Lower rib fractures may be obvious radiographically and in other instances, only confirmable with CT reformats (Fig. 22.26).
A variety of uncommon hepatic injuries have been described in abused children. Beau and colleagues noted disruption of the left hepatic duct secondary to paternal beating of a three-year-old child (167). This injury was documented at surgery by an intraoperative cholangiogram. Gornall and colleagues described a 32-month-old battered child who had suffered a complete avulsion of the common bile duct from its attachment to the duodenum (16), and Wood and associates described a case of bile duct transection due to inflicted injury (21). Goodpasture et al. describe a two-year-old abused child with thrombosis of the inferior vena cava (168). The child had a two-day history of vomiting and severe abdominal pain and had reportedly fallen out of bed that morning onto a carpeted floor. She had bruising on her face and abdomen and an ear laceration. Liver enzymes were markedly elevated. There were multiple rib fractures and abdominal CT revealed a laceration of the right hepatic lobe and inferior vena cava thrombus, confirmed sonographically two days later. Palmer and Weston reported hepatic portal vein thrombosis in a fatally abused 12-month-old infant (152). Portal venous gas may be seen with abuse; Wu and colleagues described a case of an abused two-year-old boy with portal venous gas and jejunal pneumatosis (169), and Trout and associates described a case of suspected abuse in a two-year-old with shock bowel and portal and splenic vein gas who died of his injuries (43).
With a heightened awareness of occult hepatic injuries and improved imaging technologies, it is clear that the spectrum of hepatic injuries with abuse will expand. Perez-Rossello and colleagues illustrated a hepatic laceration in an abused infant with MRI, as well as CT (Fig. 22.25) (170).
The spleen
There are few reports of splenic injury in the early literature on child abuse (171), in contrast to frequent descriptions of splenic injury with accidental trauma. Gornall and colleagues found 18 splenic injuries in 69 children with accidental abdominal injury but no splenic trauma in the 6 abused children with abdominal injuries (16). No examples of splenic injury were found in larger series of abused children with abdominal visceral injuries (13, 14, 17). The introduction of CT in the evaluation of abdominal trauma has led to increased identification of splenic injuries, although the incidence remains low. Cooper and associates found one splenic injury in 22 abused children with abdominal injuries (9). Sivit and colleagues noted 3 splenic injuries in 10 abused children with abdominal injuries documented by CT (20). Ledbetter and colleagues found no splenic injuries in 17 abused children with abdominal injuries, in contrast to splenic injuries in 47% of accidental cases (19). Wilson and associates described an infarcted spleen and left kidney in a four-month-old abused infant who presented with pneumococcal sepsis (172). Larger reviews in the recent literature cite greater numbers of splenic injury in abused children, though clearly less than hepatic injuries. In one review there were 6 splenic injuries in 84 children with inflicted abdominal injuries (7). In another review, the spleen was injured in 5 of 24 abused children with intra-abdominal trauma (22).
Imaging features
Splenic injuries may be focal or diffuse, and linear or stellate (Figs. 22.28, 22.29) (51). There may be associated perisplenic and intraperitoneal blood. In the case of avulsion of the splenic pedicle, perfusion of the spleen is absent; however, decreased or absent splenic perfusion may be noted with severe hypovolemia and an intact vascular pedicle (see Fig. 22.27). There may be associated hepatic, bowel, and pancreatic injuries. When CT images are acquired in the arterial phase, normal foci of nonenhanced parenchyma may simulate splenic laceration (Fig. 22.30). Splenic injury diagnosed by CT with IV contrast can be followed by US to document healing.

Figure 22.28 Multiple abdominal injuries. A, CT scan in an 18-month-old child with bilious vomiting shows a stellate laceration of the spleen (small arrows) and a fracture of the body of the pancreas (large arrows). B, A lower image demonstrates a hypodense duodenal hematoma (thick arrow) encroaching on the lumen of the second portion of the duodenum, trapping a rim of contrast (thin arrow) between the mass and the bowel wall. C, A lower image shows a large component of the duodenal hematoma (H) extending across the midline. Free intraperitoneal fluid (F) is present. The amylase levels were elevated and there were fractures of the thoracic spine. (Courtesy of Sheryl Sissler MD.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree