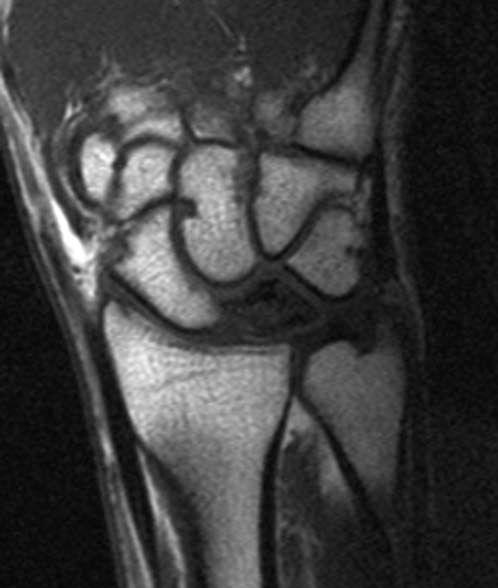
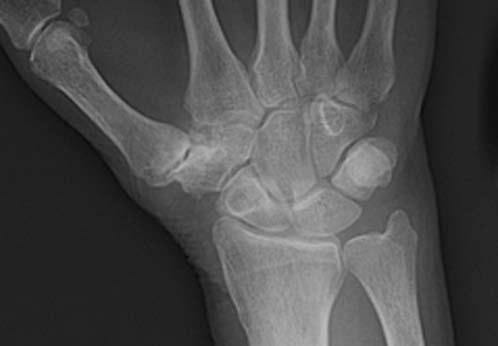
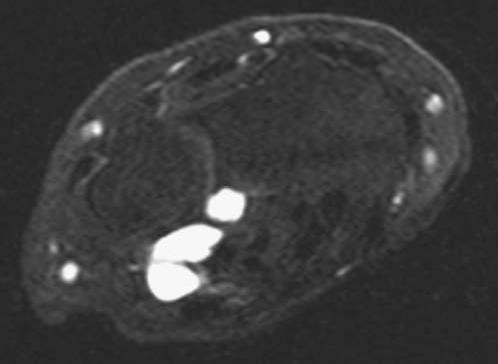
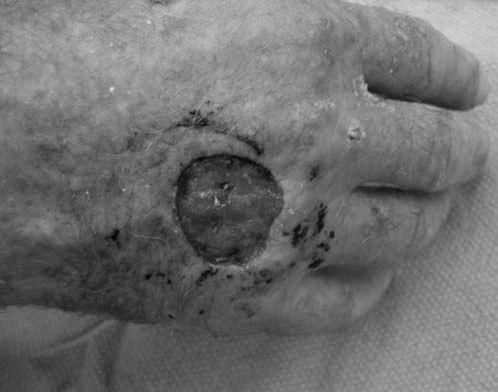
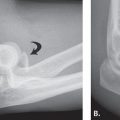
FIGURE 14.7 Eaton Stage III basal joint arthritis.
Ulnar Impaction Syndromes
History and Examination Ulnar-sided wrist pain in the absence of trauma may result from degeneration of the TFCC and proximal surface of the lunate secondary to impaction of the distal ulna against these structures (54). Impaction may result from the ulnar head articular surface or the ulnar styloid. This syndrome typically leads to the gradual onset of ulnar-sided pain aggravated by activities requiring a firm grip and pronation or ulnar deviation of the wrist. Examination reveals swelling and tenderness over the TFCC and lunotriquetral joint, which is increased with forced ulnar deviation of the wrist. There may be an associated loss of pronation and supination.
Adjunctive Studies Plain radiographs of the wrist may show degenerative arthritic changes within the ulnar head, lunate, or triquetrum. Distal prominence of the ulna relative to the radius, termed positive ulnar variance, predisposes patients to ulnar impaction and may be seen on PA views. A pronated grip radiograph may reveal a dynamic positive variance not seen on routine radiographs (55). An elongated ulnar styloid or a styloid nonunion may predispose to impaction against the triquetrum.
Arthrograms may be helpful in demonstrating degenerative tears in the TFCC. Ulnar positive variance and degenerative tears of the TFCC, however, may also be seen in asymptomatic patients. MR arthrography may be superior to conventional arthrography, because it can also reveal the pathologic results of impaction such as loss of articular cartilage and edema within the lunate or triquetrum (56).
Treatment Activity modification, splinting, and NSAIDs are the mainstays of conservative treatment of ulnar impaction. Cortisone injections may be of some use. Most procedures for impaction unresponsive to conservative treatments begin with an arthroscopy of the wrist to confirm the pathologic findings and to débride degenerative tissues. In those with ulnar neutral or negative variance, this alone may be adequate to obtain relief. In those with ulnar positive variance of only a few millimeters, arthroscopic resection of the distal 2 to 3 mm of the ulnar head may relieve impaction. If greater variance is noted, or if an intact TFCC blocks access to the ulnar head, then an open shortening of the ulna through the distal shaft may be performed (57).
Avascular Necrosis
History and Examination Idiopathic osteonecrosis of the carpus is most commonly seen in the lunate, where it is referred to as Kienböck’s disease. Patients report the gradual onset of a dull, aching pain in the dorsum of the wrist. The role of previous wrist trauma is unclear in lunate osteonecrosis, which may be the sequelae of a lunate fracture, which is occult on plain films. There may be dorsal swelling and tenderness, and wrist range of motion may become limited as the disease progresses.
Adjunctive Studies The progression of Kienböck’s disease through lunate sclerosis and collapse is typically evident on plain radiographs of the wrist. In its earliest stage, however, there are no changes on plain radiographs, but MRI images demonstrate decreased lunate signal intensity on both T1 and T2 images (58) (Fig. 14.8). MRI may also be useful to detect revascularization of the lunate after treatment, seen as increased signal on T2 images.
Treatment Immobilization and NSAIDs are standard first-line interventions, although the disease tends to progress without surgical intervention (59). Surgical treatments for degeneration limited to the lunate itself include radial shortening, lengthening of the ulna, and capitate shortening to unload the lunate. Each of these may be used alone or in combination with a vascularized bone graft to restore blood flow. If late-stage collapse of the carpus occurs, then débridement or grafting of the lunate can be performed in conjunction with one of a variety of intercarpal fusions. Radiocarpal arthritis may mandate wrist arthrodesis as a salvage procedure.
FIGURE 14.8 T1 coronal MRI demonstrating Kienböck’s disease with low signal intensity and early collapse of the lunate.
Inflammatory Disorders
Atypical Infections
History and Examination Most acute hand infections are the result of bacteria such as Staphylococcus aureus and have a rapid initial onset and either resolve quickly with treatment or rapidly destroy tissue if left untreated. Infections that persist over long periods of time may represent osteomyelitis or atypical infections from mycobacteria or fungi. History and examination are the same as described for the evaluation of acute infections, but more emphasisis placed on history of environmental exposures to determine the causative organism.
Mycobacteria may produce chronic hand infections (60). Mycobacterium tuberculosis is uncommon, but Mycobacterium marinum is commonly seen in patients who have contact with fish or seawater. Mycobacterium avium–intracellulare is usually seen in immunocompromised patients such as those with HIV and may be associated with exposure to poultry or contaminated soil. Both of these organisms may produce cellulitis, osteomyelitis, or septic arthritis with sinus tracts from chronic synovitis.
Fungi frequently produce smoldering, low-level infections that are difficult to diagnose (61). Candida albicans is responsible for the majority of chronic paronychial infections and is seen in those with diabetes and those whose hands are frequently immersed in water. Fingernails may demonstrate thickening and ridging (onychomycosis). Sporothrix schenckii may be inoculated through rose bush thorns, leading to a local papule and proximal lymphatic spread. Coccidioides immitis is endemic to the southwestern United States and typically affects the synovium of the hand through hematogenous spread from the lungs. Histoplasma capsulatum is endemic to the Ohio and Mississippi river basins and may lead to a caseating tenosynovitis. Blastomycosis occurs in the same region of the United States but leads to cutaneous nodules and ulceration.
Adjunctive Studies Fungal and mycobacterial infections do not typically grow on routine cultures; therefore, a high level of suspicion and knowledge of the appropriate studies is therefore necessary. Mycobacterial infections aside from tuberculosis do not typically lead to positive skin tests. Tissue biopsies are taken so that microscopic evaluation of granulomas may be performed. Cultures require special agar plating at nonstandard temperatures and may take up to 6 weeks for final results. Similarly, fungal cultures may require several weeks for accurate results, but in cases such as Histoplasmosis, a microscopic examination after potassium hydroxide preparation may reveal characteristic hyphae. In all cases, routine radiographs of the hand are warranted to evaluate for osteomyelitis or septic arthritis. MRI scans may also show marrow edema from osteomyelitis while demonstrating the extent of any synovitis.
Treatment Atypical infections are ideally treated with the assistance of an infectious disease specialist. Mycobacterial infections are treated by minocycline and trimethoprim–sulfamethoxazole for M. marinum or clarithromycin, rifabutin, and ethambutol for M. avium–intracellulare. In either condition, débridement and synovectomy may be necessary in longstanding or severe cases. Fungal infection with C. albicans may be treated effectively with topical or oral agents such as itraconazole, but may require marsupialization of the nail if the antifungals are not effective. The deeper fungal infections that cause synovitis or osteomyelitis typically require aggressive débridement as well as amphotericin B.
Repetitive Strain Injuries
History and Examination Repetitive strain injuries are characterized by tendonitis and tenosynovitis as previously described for acute overuse injuries. Persistent inflammation may lead to the degeneration of tendon origins and insertions or the formation of dense scar tissue, which inhibits tendon excursion. Additionally, chronic inflammation of tissues around the median, radial, or ulnar nerves may lead to nerve compression syndromes with pain, paresthesias, and sensory or motor loss in the hands. Examination of the entire upper extremity is essential, because the inflammation is frequently multifocal.
Adjunctive Studies Electromyography and nerve conduction studies may confirm compression of peripheral nerves. MRI may reveal degenerative scar tissue within tendon origins or inflamed synovium surrounding or invading tendons.
Treatment Most inflammation from repetitive strain injuries will respond to a prolonged course of activity modification, ergonomic improvements in the work-place, rest of the inflamed tissues, splinting, NSAIDs, and cortisone injections. Physical or occupational therapy for range-of-motion and strengthening exercises as well as modalities such as cold packs, ultrasound, and iontophoresis or phonophoresis may also help to diminish inflammation and scarring. Inflammation that is unresponsive to prolonged conservative treatment may require surgical débridement, and documented nerve compression may benefit from surgical decompression at the same setting.
Immune-mediated Inflammatory Disorders
History and Examination The hands and wrist are frequently involved in immune-mediated inflammatory disorders such as rheumatoid arthritis, systemic lupus erythematosus (SLE), psoriatic arthritis, scleroderma, and the crystalline arthropathies (gout and pseudogout). Inflammatory arthritis in the hands may be the first presentation for each of these disorders. The classic diagnostic criteria for rheumatoid arthritis are morning stiffness of longer than 1 hour; involvement of three or more joints simultaneously; symmetric joint involvement; arthritis of the wrist, MCP, or PIP joints for longer than 6 weeks; rheumatoid nodules; elevated rheumatoid factor (RF); and subchondral erosions and osteopenia on radiographs (62). The diagnosis of rheumatoid arthritis is made if four of the seven criteria are met. Common findings on examination are radial deviation and supination of the carpus with secondary prominence of the ulnar head, ulnar deviation and subluxation of the MCP joints, and swan-neck deformities of the PIP joints (63).
Manifestation of SLE in the hands is usually limited to the soft tissues, affecting women in their second and third decades. Swan-neck deformities and MCP instability are common findings. Raynaud’s phenomenon is frequently seen in SLE, but may also be seen in a more severe form in scleroderma (64). Calcinosis of the fingers with shiny, thick skin and fingertip necrosis are common findings in scleroderma. Both SLE and scleroderma frequently have multiple systemic manifestations. Psoriatic arthritis develops in approximately 5% of patients with the typical scaly, erythematous skin lesions. The IP joints in psoriatic arthritis are subject to severe bony erosion and soft tissue destruction leading to instability (65).
The crystalline arthropathies, gout and pseudogout, occur as the result of synovial deposition of sodium urate and calcium pyrophosphate crystals. The subsequent inflammatory reaction to the crystals leads to severe pain, swelling, and erythema within the joints, often mimicking septic arthritis. It frequently appears in males in their fifth and sixth decades (65).
Adjunctive Studies Most patients will have an elevated ESR and CRP as a result of the inflammation from these disorders. More specific laboratory findings include a positive RF titer in 80% of patients with adult-onset rheumatoid arthritis and elevated antinuclear antibodies and double-stranded DNA in SLE (60, 62). There are no specific laboratory studies for scleroderma, psoriatic arthritis, gout, or pseudogout, but gout may be associated with elevated uric acid levels (65).
Radiographs in rheumatoid arthritis show the deformities described here with regional osteoporosis and marginal erosions of the joint surfaces (Fig. 14.9). SLE manifests as joint subluxation and dislocation without significant articular erosion. Interphalangeal joints in psoriatic arthritis demonstrate the most severe erosions with a characteristic “pencil-in-cup” appearance of involved joints. Gout produces punched-out articular erosions along with calcium build-up or tophi in the periarticular soft tissues. Pseudogout is usually less destructive than gout and often leaves pathognomonic calcifications within the TFCC known as chondrocalcinosis (65). MRI may be used in any of these disorders to more clearly delineate the site and extent of synovitis as well as the status of involved ligaments. Bone marrow edema appears to be predictive of future periarticular erosions in each of the inflammatory arthritides (66).
FIGURE 14.9 Typical radiograph in advanced rheumatoid arthritis with radial deviation of the carpus, ulnar deviation, and dislocation of the metacarpophalangeal joints, bony erosions, and thumb boutonniére deformity.
Joint aspiration is usually performed both to confirm the diagnosis and to rule out septic arthritis. Negatively birefringent, yellow sodium urate crystals are characteristic of gout, whereas positively birefringent, purple calcium pyrophosphate crystals confirm the diagnosis of pseudogout. Rheumatoid arthritis, SLE, and psoriatic arthritis do not have any specific synovial findings other than elevated synovial white blood counts and the absence of infectious organisms.
Treatment Treatment should proceed in conjunction with a referral to a rheumatologist for appropriate medical management. In the noncrystalline inflammatory arthropathies, this may include prednisone, methotrexate, and newer “disease-modifying” drugs that limit inflammation and the progression of the disease. Splinting and NSAIDs also play a major role in conservative treatment. Surgical treatment of these disorders involves synovectomy for pain relief and to limit joint and tendon destruction as well as tendon transfers to replace the function of damaged tendons. Arthrodesis is often performed in painful IP joints, whereas resection or implant arthroplasties are performed in MCP joints. The ulnar head may be resected if prominent in rheumatoid arthritis. Swan-neck and boutonniére deformities are addressed with a variety of soft tissue procedures.
Vascular spasm and occlusion in scleroderma may be medically managed with calcium channel blockers and nitropaste as well as surgical sympathectomy (64). Amputations are occasionally necessary in refractory cases. Gout may be medically managed with colchicine and indomethacin for acute attacks and allopurinol to prevent recurrence (65). Occasionally, débridement of a tophus is required if infection or skin erosion occurs. Pseudogout as well as all of the previously mentioned disorders affecting the joints can be treated with intraarticular cortisone injections if septic arthritis has been ruled out of the differential diagnosis.
Hand and Wrist Masses
Benign Soft Tissue Lesions
History and Examination The great majority of masses appearing within the wrist and hand are benign. Of these, 50% to 70% are ganglions (67). Other common benign masses include giant cell tumors arising from tendon sheaths, epidermal inclusion cysts, lipomas, and nerve sheath tumors. Vascular lesions and osteophytes may also present as palpable masses and are dealt with elsewhere in this Chapter. Ganglion cysts are firm, synovial fluid-filled cysts arising from joints or tendon sheaths. Dorsal wrist ganglions typically arise from the scapholunate interval, whereas volar wrist ganglions arise from the radiocarpal or scaphotrapezial joints. Volar retinacular cysts arise from the MCP joint or flexor tendon sheath. These cysts may cause symptoms related to local mass effect, including tenderness, pain with joint range of motion, triggering of flexor tendons, or compression of the ulnar nerve within Guyon’s canal. Mucous cysts from the DIP joint may compress the nail germinal matrix leading to nail plate deformities. Ganglions are typically firm and fixed to the underlying tissues but not the skin and will transilluminate.
Giant cell tumors of the tendon sheath are also usually fixed to underlying tissues, but they are solid and do not transilluminate. Epidermal inclusion cysts are also firm and similar in size to ganglions, but they are adherent to the skin and do not appear in consistent locations or transilluminate. Lipomas are slow-growing, usually painless benign growths of fatty tissue that are typically larger and softer than other hand masses. They may compress adjacent structures. Neurofibromas and schwannomas both arise from the nerve sheath, but schwannomas usually grow outward, whereas neurofibromas grow inward and may be solitary or part of a systemic neurofibromatosis. They may create symptoms from axonal compression, and the multifocal neurofibromas seen in von Recklinghausen’s disease are at risk for malignant degeneration. Schwannomas, on the other hand, are not associated with malignant degeneration.
Adjunctive Studies Simple ganglion cysts, giant cell tumors, inclusion cysts, and small lipomas with typical presentations typically do not require any imaging studies. Plain radiographs may be performed to document the soft tissue shadows or to look for joint degeneration, which may lead to ganglion formation. If any aspect of the mass or the patient’s symptoms are atypical, however, then an MRI is warranted to obtain the best evaluation of the soft tissue characteristics and extent of the mass (Fig. 14.10).
Treatment Each of these masses may be treated with excisional biopsy. Ganglion excision must include the stalk and the capsular rent responsible for the cyst formation. Typical dorsal ganglion cysts may also be treated with aspiration, although there is a very high recurrence rate (67).
FIGURE 14.10 Axial MRI of the wrist with an atypical volar–ulnar location of a multilobular ganglion cyst.
Benign Bone Lesions
History and Examination Common benign bone lesions in the distal radius or ulna and hand include enchondromas, unicameral and aneurysmal bone cysts (UBC and ABC), osteochondromas, osteoid osteomas, and giant cell tumors. Enchondromas, bone cysts, and giant cell tumors are commonly painless and may present as incidental findings after nearby fractures or pathologic fractures through the lesion. ABCs, giant cell tumors, and osteochondromas may also present with swelling that is either painless or tender as a result of compression of overlying structures. Osteoid osteomas are frequently associated with severe aching pain, which wakes the patient at night but is responsive to anti-inflammatory medications. Most of these lesions appear in children or young adults.
Adjunctive Studies Radiographs are usually diagnostic because of the characteristic appearance of each lesion. Enchondromas appear in the metadiaphyseal region of the phalanges and metacarpals with thinning of the cortex, stippled calcifications of the matrix, and minimal expansion of the cortex. UBCs have no calcifications and are mildly expansile, whereas ABCs are often seen in the distal radius as multiloculated, highly expansile lucent metaphyseal lesions that do not cross the physis. Giant cell tumors commonly appear in the distal radius, but they are less expansile than ABCs and frequently extend across the physeal scar to the subchondral bone. Osteoid osteomas will have a small sclerotic nidus surrounded by a lucent halo. The nidus may be better visualized on CT scans and will have high uptake on bone scans (68). Osteochondromas are unique among these lesions in that they extend from the metaphyseal cortex outward, away from the bone and toward the diaphysis. They have a cartilaginous cap, which is visible on CT scans.
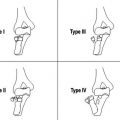
Treatment Enchondromas are treated with curettage and bone grafting. Malignant degeneration is uncommon in solitary lesions but common in disorders with multiple lesions such as Ollier’s disease or especially Maffucci’s syndrome. UBCs often resolve spontaneously, but those at risk for fracture may be treated with irrigation and injection of steroid or bone graft. ABCs tend to recur unless treated with complete excision and bone grafting. Giant cell tumors are similarly treated with wide excision with preservation of the subchondral bone. Recurrence may be minimized with adjuvant cementation, cryotherapy, or application of phenol (68). Osteochondral allografts may be required for joint reconstruction. Osteoid osteomas are treated with curettage or radiofrequency ablation.
Malignant Soft Tissue Lesions
History and Examination Squamous cell carcinoma, basal cell carcinoma, and malignant melanoma are commonly seen in the skin and nails of the hand. Common malignant lesions of the deep soft tissues of the hand include epithelioid sarcomas, synovial sarcomas, and malignant fibrous histiocytomas (MFH). Squamous cell carcinoma is the most common hand malignancy and tends to occur in elderly, fair-skinned people of northern European descent who have significant sun exposure or chronic, nonhealing wounds (68). It presents as a scaly red plaque or an ulcer with raised edges (Fig. 14.11). Basal cell carcinomas appear in the same demographic group, but typically appear as slow-growing raised, erythematous plaques with pearly borders. Metastases are rare. Malignant melanoma is a more aggressive tumor, frequently presenting as a pigmented lesion of the fingertips and nail bed, which develops an irregular color, border, or surface texture.
Both epithelioid and synovial cell sarcomas often appear as painless, slow-growing masses or nodules within the muscles of the hand in males 20 to 40 years old. They may be adherent to underlying structures and extend along tissue planes. Patients with MFH are typically older with the mass appearing in the deep tissues of the hand and frequently invading bone.
FIGURE 14.11 Squamous cell cancer presenting as a nonhealing dorsal hand ulcer with raised, rounded edges.
Adjunctive Studies A full staging workup is usually indicated, including local radiographs, an MRI including the entirety of all involved compartments, CT scanning of the chest, urinalysis, complete blood count, electrolytes, alkaline phosphatase, and liver function studies (69). Further testing such as bone scans may be indicated based on a review of the patient’s symptoms. Incisional biopsies are performed to confirm the diagnosis. For squamous and basal cell carcinomas, biopsy and excision can be performed through a single procedure using the Mohs’ technique of sequential tangential excision and histologic evaluation (70). Lymph node biopsy in melanomas may improve early recognition of metastases.
Treatment Wide or radical excision is indicated for all of these lesions, which in the hand often necessitates amputation. Adjuvant treatments are given in conjunction with a medical oncologist and may include radiation treatments or chemotherapy.
Malignant Bone Lesions
History and Examination Malignant bone lesions such as chondrosarcoma, osteosarcoma, and Ewing’s sarcoma are extremely rare in the hand. Metastases may appear from lung, breast, colon, and renal cancers, but are similarly rare (68). Chondrosarcoma may appear in elderly patients as the result of malignant degeneration of an enchondroma. They are typically heralded by the new onset of swelling and pain in a previously asymptomatic lesion. Ewing’s sarcoma and osteosarcoma appear in children and adolescents. Both present as a rapidly growing, painful lesion. Ewing’s sarcoma may also be accompanied by fever.
Adjunctive Studies Plain radiographs of a chondrosarcoma will show erosion and bony or periosteal reaction around a lytic region with a matrix of stippled calcifications. Osteosarcoma will typically show bone formation in the soft tissues in association with erosion of the cortex. Ewing’s sarcoma has a permeative, destructive pattern with a major periosteal reaction. Metastases usually appear as small destructive lesions within the distal phalanges. Regional MRI and full staging studies are indicated for all of these lesions, and incisional biopsy will usually confirm the diagnosis (68).
Treatment Wide excision alone or amputation is frequently curative for the relatively slow-growing chondrosarcomas. Osteosarcomas and Ewing’s sarcomas are much more aggressive and require neoadjuvant and adjuvant chemotherapy in association with wide excision. Radiation may also be used to treat Ewing’s sarcoma. Metastases are treated based on the primary tumor type.
Nerve Compression Syndromes
Median Nerve Compression
History and Examination Compressive neuropathies may lead to pain, paresthesias, sensory loss, and motor loss within the hand and wrist. The actual site of compression of the nerve may be as far proximal as the cervical root level, the thoracic outlet, or at multiple potential points of compression along the length of the nerve. Symptoms that radiate from the neck to a specific dermatome suggest a cervical root compression. Symptoms involving the entire arm and hand may result from compression of the brachial plexus at the thoracic outlet. There are multiple potential sites of compression of the median, radial, and ulnar nerves, which innervate the hand.
Median nerve compression may occur proximally at the ligament of Struthers, which passes from the humerus to the medial epicondyle, the lacertus fibrosis, the two heads of the pronator teres, and the proximal edge of the flexor digitorum superficialis. Distal compression may occur within the carpal canal and is known as carpal tunnel syndrome. Proximal compression produces pain and paresthesias radiating to the hand as well as tenderness and a positive Tinel’s sign over the nerve as it passes by the antecubital fossa. There may be weakness of the FCR and digital flexors in addition to the thenar muscles. Compression involving only the anterior interosseous branch will produce weakness of the forearm muscles without sensory loss. Carpal tunnel syndrome, however, will have pain, tenderness, and positive Tinel’s, Phalen’s, and carpal compression signs at the carpal canal with motor loss of the thenar muscles only (6).
Adjunctive Studies Electromyography and nerve conduction studies may be used to confirm the diagnosis of median nerve compression and the suspected site of compression. If the diagnosis is still in question, or if there are concerns regarding extensive synovitis, nerve sheath tumors, or other masses within the carpal canal, then MRI of the wrist can visualize the median nerve and any sites of compression (71).
Treatment Conservative treatment involves rest, night splinting of the wrist, and possible cortisone injections into the carpal canal. If these fail to relieve symptoms or if the sensory and motor changes are severe, then the nerve is surgically decompressed at the likely site of compression with open or endoscopic techniques (72).
Ulnar Nerve Compression
History and Examination Potential sites of ulnar nerve compression are a fascial band from the triceps known as the Arcade of Struthers, the medial intermuscular septum, the cubital tunnel, Osborne’s ligament between the heads of the FCU, and within Guyon’s canal at the wrist. Proximal compression at the elbow will lead to weakness of the FCU and FDP to the ring and small fingers in addition to the hand intrinsic muscles. Sensory loss will involve the entire ulnar side of the hand. Compression at the wrist, which leads to weakness of the intrinsics but spares the FDP proximally, may lead to clawing of the ring and small fingers in severe cases. Compression at the wrist also spares the dorsal hand sensation, because the dorsal sensory branch bifurcates proximal to Guyon’s canal. Examination may reveal tenderness and a positive Tinel’s sign at the elbow or wrist corresponding to the site of compression. Flexion of the elbow may lead to palpable subluxation of the nerve in some cases.
Adjunctive Studies
Like in compression of the median nerve, electrodiagnostic studies may confirm the site of compression, and an MRI may be warranted in atypical cases (71). Occasionally, a ganglion cyst or ulnar artery aneurysm may be present in Guyon’s canal, leading to compression of the nerve.
Treatment Treatment of ulnar nerve compression at the elbow that does not respond to NSAIDs, rest, and splinting the elbow in extension at night involves surgical decompression of all potential sites of compression. Decompression usually involves anterior transposition of the nerve into a subcutaneous or submuscular pocket. Compression at the wrist is treated with open decompression of Guyon’s canal and excision of any associated masses.
Radial Nerve Compression
History and Examination The radial nerve may be compressed within the radial tunnel, which extends from a series of fibrous bands anterior to the radial head to the distal edge of the supinator. There are multiple potential sites of compression within this region. Compression may also occur distally as the superficial sensory branch emerges from the radial forearm fascia. This is known as Wartenberg’s syndrome (73). Proximal compression may manifest as a painful phenomenon without motor or sensory changes known as radial tunnel syndrome or as a pure motor loss without pain or sensory changes resulting from compression of the posterior interosseous nerve (PIN). PIN syndrome will spare the brachioradialis, anconeus, and ECRL, which are more proximally innervated. Radial tunnel syndrome will have associated tenderness at the radial tunnel with distal radiation but without paresthesias. Wartenberg’s syndrome will have tenderness and a positive Tinel’s sign over the nerve at the distal radial forearm with burning paresthesias on the dorsum of the hand.
Adjunctive Studies Electrodiagnostics may be helpful in the radial nerve compression syndromes. Response to local anesthetic injections may be confirmatory of the site of compression. MRI of the forearm is often obtained to rule out any masses that may be compressing the nerve in PIN syndrome (71).
Treatment Rest, splinting, and NSAIDs are again the mainstays of conservative treatment. Symptoms that persist for longer than 3 months are usually an indication for surgical decompression.
Vascular Disorders
Raynaud’s Phenomenon
History and Examination Raynaud’s phenomenon manifests as cold-induced vasospasm of the digits, resulting in pain, pallor, and paresthesias with intense hyperemia after rewarming and restoration of blood flow. This may be an isolated, idiopathic phenomenon known as Raynaud’s disease or a secondary phenomenon known as Raynaud’s syndrome (41). Severe cases may lead to trophic changes of the skin and even digital tip ulcerations.
Adjunctive Studies The diagnosis of primary Raynaud’s disease is made after clinical evaluation and laboratory studies have ruled out other related conditions such as rheumatoid arthritis, SLE, and cryoglobulinemia. Arteriograms and MR angiography may play a role in ruling out proximal arterial occlusions or systemic vasculitis (41).
Treatment Treatment in Raynaud’s syndrome is directed toward the inciting condition. Smoking cessation and maintaining warm hands with appropriate clothing and gloves are appropriate initial steps. Nitro-paste, calcium channel blockers, and sympathetic blocks may decrease arterial tone. Trental may improve red blood cell deformation, whereas dextran may act as a volume expander. In refractory cases, surgical sympathectomy at the stellate ganglion or digital sympathectomy may be attempted (41).
Arterial Aneurysm and Pseudoaneurysm
History and Examination Aneurysms may occur in the hand and wrist as a result of trauma to the arterial wall. Blunt trauma typically produces a true aneurysm in which the entire arterial wall is dilated. Penetrating trauma may separate the layers of the arterial wall, allowing blood to create a false passage in the wall known as a pseudoaneurysm. Both types of aneurysms present as painful, pulsatile masses that may compress nearby structures. Palpation may reveal a thrill, and auscultation may reveal a bruit in larger aneurysms. Allen’s test may reveal alterations in blood flow and the adequacy of redundant flow.
Adjunctive Studies Doppler examination and segmental arterial pressures may be helpful. Duplex ultrasound and MRI may be helpful in differentiating aneurysms from other masses such as ganglia, which may seem pulsatile due to their proximity to an artery (74).
Treatment Aneurysms may be resected with interposition vein graft. In cases of exceptional redundant flow, the arterial ends may be ligated.
Vascular Malformations
History and Examination Vascular malformations in the hand typically present with pain, swelling, compressive effects on nearby structures, and temperature-related changes in size or color. Glomus tumors are small vascular tumors that appear in the nail bed and deform the nail plate. Pyogenic granulomas are small, red cutaneous growths, whereas hemangiomas are made up of residual mesodermal tissues filled with blood. They may appear within the skin or as subcutaneous masses. Arteriovenous malformations (AVMs) are typically painful bundles of vessels that communicate between arteries and veins while bypassing the capillaries. Flow through an AVM may be significant enough to steal blood flow from distal tissues and to cause cardiac hypertrophy and strain (41).
Adjunctive Studies Plain radiographs are useful to evaluate for body erosions resulting from the masses. Arteriograms and MRIs can visualize the extent of AVMs, whereas Duplex ultrasound can characterize the flow patterns.
Treatment Symptomatic glomus tumors are treated with removal of the nail plate, excision of the tumor, and repair of the nail bed. Pyogenic granulomas are treated with cauterization or excision. Hemangiomas present at birth are treated with observation as many will resolve spontaneously (7). Those that persist may be excised unless they are too extensive. The potential for significant blood loss makes excision of AVMs quite hazardous. Surgery is therefore reserved only for lesions that lead to persistent pain, frequent bleeding, distal steal phenomena, or cardiac side effects. Conservative treatment consists of compressive gloves or sleeves.
References
1. Grundberg AB, Reagan DS. Pathologic anatomy of the forearm: intersection syndrome. J Hand Surg. 1985;10:299–302.
2. Mannerfelt L. Rupture of the extensor pollicis longus tendon after Colles fracture and by rheumatoid arthritis. J Hand Surg (Br). 1990;15:49–50.
3. Geissler WB. Intracarpal soft-tissue lesions associated with an intra-articular fracture of the distal end of the radius. J Bone Joint Surg Am. 1996;78:357–365.
4. Reagan DS, Linscheid RL, Dobyns JH. Lunotriquetral sprains. J Hand Surg. 1984;9:502–514.
5. Bowers WH. The distal radioulnar joint. In: Green DP, Hotch-kiss RN, Pederson WC, eds. Green’s Operative Hand Surgery.4th ed. Philadelphia, Pa: Churchill Livingstone; 1999:1015–1019.
6. Marx RG, Bombardier C, Wright JG. What do we know about the reliability and validity of physical examination tests used to examine the upper extremity? J Hand Surg (Am). 1999;24:185–193.
7. Phillips CS, Murphy MS. Vascular problems of the upper extremity: a primer for the orthopaedic surgeon. J Am Acad Orthop Surg. 2002;10:401–408.
8. Han SK, Lee BI, Kim WK. Topical and systemic anticoagulation in the treatment of absent or compromised venous outflow in replanted fingertips. J Hand Surg (Am). 2000;25:659–667.
9. Mannerfelt L. Attrition ruptures of flexor tendons in rheumatoid arthritis caused by bony spurs in the carpal tunnel. J Bone Joint Surg Br. 1969;51:270–277.
10. Bencardino JT. MR imaging of tendon lesions of the hand and wrist. MRI Clinics. 2004;12:333–348.
11. Garberman SF, Diao E, Peimer CA. Mallet finger: results of early versus delayed closed treatment. J Hand Surg (Am). 1994;19:850–852.
12. Newport ML. Extensor tendon injuries in the hand. J Am Acad Orthop Surg. 1997;5:59–66.
13. Feldon P, Terrono AL, Nalebuff EA, et al. Rheumatoid arthritis and other connective tissue diseases. In: Green DP, Hotchkiss RN, Pederson WC, eds. Green’s Operative Hand Surgery. 4th ed. Philadelphia, Pa: Churchill Livingstone; 1999:1670–1680.
14. Bishop AT, Beckenbaugh RD. Fracture of the hamate hook. J Hand Surg (Am). 1988;13:135–139.
15. Breitenseher MJ, Metz VM, Gilula LA. Radiographically occult scaphoid fractures: value of MR imaging in detection. Radiology. 1997;203:245–250.
16. Trumble TE. Hand fractures. In: Trumble TE, ed. Principles of Hand Surgery and Therapy. Philadelphia, Pa: WB Saunders Company; 2000:72–77.
17. Heyman P. Injuries to the ulnar collateral ligament of the thumb metacarpophalangeal joint. J Am Acad Orthop Surg. 1997;5:224–229.
18. Bowers WH, Hust LC. Gamekeeper’s thumb. Evaluation by arthrography and stress roentgenography. J Bone Joint Surg Am. 1977;59:519–524.
19. O’Callaghan BI, Kohut G, Hoogewoud H-M. Gamekeeper thumb: identification of the Stener lesion with US. Radiology. 1994;192:477–480.
20. Louis DS, Buckwalter KA. Magnetic resonance imaging of the collateral ligaments of the thumb. J Hand Surg (Am). 1989;14:739–741.
21. Stern PJ. Fractures of the metacarpals and phalanges. In: Green DP, Hotchkiss RN, Pederson WC, eds. Green’s Operative Hand Surgery. 4th ed. Philadelphia, Pa: Churchill Livingstone; 1999:727–729.
22. Simonian PT, Trumble TE. Traumatic dislocation of the thumb carpometacarpal joint: early ligamentous reconstruction versus closed reduction and pinning. J Hand Surg (Am). 1996;21:802–806.
23. Linsheid RL, Dobyns JH, Beaubout JW, et al. Traumatic instability of the wrist: diagnosis, classification, and pathomechanics. J Bone Joint Surg Am. 1972;54:1612–1632.
24. Mayfield JK. Patterns of injury to carpal ligaments: a spectrum. Clin Orthop Relat Res. 1984;187:36–42.
25. Watson HK, Ashmead D IV, Makhlouf MV. Examination of the scaphoid. J Hand Surg (Am). 1988;13:657–660.
26. Lichtman DM, Schneider JR, Swafford AR. Ulnar midcarpal instability. Clinical and laboratory analysis. J Hand Surg. 1981;6:515–523.
27. Gundry CR, Kursunoglu-Brahme S, Schwaighofer B, et al. Is MRI better than arthrography for evaluating the ligaments of the wrist? In vitro study. AJR Am J Roentgenol. 1990;154:337–341.
28. Scheck RJ, Romagnolo A, Hierner R, et al. The carpal ligaments in MR arthrography of the wrist: correlation with standard MRI and wrist arthroscopy. J Magn Reson Imaging. 1999;9:468–474.
29. Zanetti M, Bram J, Hodler J. Triangular fibrocartilage and intercarpal ligaments of the wrist: does MR arthrography improve standard MRI. J Magn Reson Imaging. 1997;7:590–594.
30. Wolfe SW. Scapholunate instability. J Am Soc Surg Hand. 2001;1: 45–60.
31. Butterfield WL, Joshi AB, Lichtman D. Lunotriquetral injuries. J Am Soc Surg Hand. 2002;2:195–203.
32. Calandruccio JH. Proximal row carpectomy. J Am Soc Surg Hand. 2001;1:112–122.
33. White RE, Omer GE Jr. Transient vascular compromise of the lunate after fracture–dislocation or dislocation of the carpus. J Hand Surg (Am). 1984;9:181–184.
34. Tomaino MM, Rubin DA. The value of the ‘pronated grip radiograph’ in assessing for dynamic ulnar positive variance: a case report. Am J Orthop. 1999;3:180–181.
35. Wechsler RJ, Webbe MA, Rifkin MD, et al. Computed tomography diagnosis of distal radioulnar subluxation. Skeletal Radiol. 1987;16:1–5.
36. Pederzini L, Luchetti R, Soragni O, et al. Evaluation of the triangular fibrocartilage complex tears by arthroscopy, arthrography, and magnetic resonance imaging. Arthroscopy. 1992;8:191–197.
37. Cerofolini E, Luchetti R, Pederzini L, et al. MR evaluation of triangular fibrocartilage complex tears in the wrist: comparison with arthrography and arthroscopy. J Comput Assist Tomogr. 1990;14:963–967.
38. Oneson SR, Timins ME, Scales LM, et al. MR imaging diagnosis of triangular fibrocartilage pathology with arthroscopic correlation. AJR Am J Roentgenol. 1997;168:1513–1518.
39. Adams BD, Berger RA. An anatomic reconstruction of the distal radioulnar ligaments for posttraumatic distal radioulnar joint instability. J Hand Surg (Am). 2002;27:86–92.
40. Koman LA, Urbaniak JR. Ulnar artery thrombosis. Hand Clin. 1985;1:311–325.
41. Gupta A. Basic vascular pathophysiology of the hand, wrist, and forearm. In: Berger RA, Weiss AC. eds. Hand Surgery. Philadelphia, Pa: Lippincott Williams & Wilkins; 2004:49–67.
42. Burton RI, Pellegrini VD Jr. Surgical management of basal joint arthritis of the thumb. Part II. Ligament reconstruction with tendon interposition arthroplasty. J Hand Surg (Am). 1986;11:324–332.
43. Diao E. Trapeziometacarpal arthritis: trapezium excision and ligament reconstruction not including the LRTI arthroplasty. Hand Clin. 2001;17:223–236.
44. Berger RA. A technique for arthroscopic evaluation of the first carpometacarpal joint. J Hand Surg (Am). 1997;22:1077–1080.
45. Linscheid RL, Weber ER. Scaphoid fractures and nonunions. In: Cooney WP, Linscheid RL, Dobyns JH, eds. The Wrist: Diagnosis and Operative Treatment. St. Louis, Mo: CV Mosby; 1998.
46. Perlik PC, Guilford WB. Magnetic resonance imaging to assess vascularity of scaphoid nonunions. J Hand Surg (Am). 1991; 16:479–484
47. Watson HK, Ballet FL. The SLAC wrist: scapholunate advanced collapse pattern of degenerative arthritis. J Hand Surg. 1984;9:358–365.
48. Shin AY, Bishop AT. Vascularized bone grafts from the distal radius for disorders of the carpus. J Am Soc Surg Hand. 2002;2:181–194.
49. Cohen MS, Kozin SH. Degenerative arthritis of the wrist: proximal row carpectomy versus scaphoid excision and 4-corner arthrodesis. J Hand Surg (Am). 2001;26:94–104.
50. Jarvik JG, Kliot M, Maravilla KR. MR nerve imaging of the wrist and hand. Hand Clin. 2000;16:13–24.
51. Eaton RG, Glickel SZ. Trapeziometacarpal osteoarthritis: staging as a rationale for treatment. Hand Clin. 1987;3:455–471.
52. Eaton RG, Littler JW. Ligament reconstruction for the painful thumb carpometacarpal joint. J Bone Joint Surg Am. 1973;55:1655–1666.
53. Bamberger HB, Stern P, Keifhaber TR, et al. Trapeziometacarpal arthrodesis: a functional evaluation. J Hand Surg (Am). 1992;17:605–611.
54. Friedman SL, Palmer AK. The ulnar impaction syndrome. Hand Clin. 1991;7:295–310.
55. Tomaino MM. The importance of the pronated grip x-ray view in evaluating ulnar variance. J Hand Surg (Am). 2000;25:352–357.
56. Cerezal L, del Pinal F, Abascal F. MR imaging findings in ulnar-sided wrist impaction syndromes. Magn Reson Imaging Clin N Am. 2004;12:281–299.
57. Pomerance J. Arthroscopic débridement and/or ulnar shortening osteotomy for TFCC tears. J Am Soc Surg Hand. 2002;2: 95–101.
58. Trumble TE, Irving JI. Histologic and magnetic resonance imaging correlations in Kienböck’s disease. J Hand Surg (Am). 1990;15:879–884.
59. Beckenbaugh RD, Shives TC, Dobyns JH, et al. Kienböck’s disease: the natural history of Kienböck’s disease and consideration of lunate fractures. Clin Orthop Relat Res. 1980;149:98–106.
60. Kozin SH, Bishop AT. Atypical Mycobacterium infections of the upper extremity. J Hand Surg (Am). 1994;19:480–487.
61. Trumble TE, Hashisaki P. Hand infections. In: Trumble TE, ed. Principles of Hand Surgery and Therapy. Philadelphia, Pa: WB Saunders; 2000:214–221.
62. Anderson RJ. Rheumatoid arthritis: clinical and laboratory features. In: Klippel JH, ed. Primer on the Rheumatic Diseases. 11th ed. Atlanta, Ga: Arthritis Foundation; 1997:161–167.
63. Feldon P, Terrono AL, Nalebuff EA, et al. Rheumatoid arthritis and other connective tissue diseases. In: Green DP, Hotchkiss RN, Pederson WC, eds. Green’s Operative Hand Surgery. 4th ed. New York, NY: Churchill Livingstone; 1999:1651–1739.
64. Wigley FM. Systemic sclerosis and related syndromes. In: Klippel JH, ed. Primer on the Rheumatic Diseases. 11th ed. Atlanta, Ga: Arthritis Foundation; 1997:267–272.
65. Ali Y. Crystalline arthritis and other arthritides. In: Berger RA, Weiss AC, eds. Hand Surgery. Philadelphia, Pa: Lippincott Williams & Wilkins; 2004:1241–1252.
66. Jbara M, Patnana M, Kazmi F, et al. MR imaging: arthropathies and infectious conditions of the elbow, wrist, and hand. Magn Reson Imaging Clin N Am. 2004;12:361–379.
67. Angelides AC. Ganglions of the hand and wrist. In: Green DP, Hotchkiss RN, Pederson WC, eds. Green’s Operative Hand Surgery. 4th ed. New York, NY: Churchill Livingstone; 1999:2171–2183.
68. Athanasian EA. Bone and soft tissue tumors. In: Green DP, Hotchkiss RN, Pederson WC, eds. Green’s Operative Hand Surgery. 4th ed. New York, NY: Churchill Livingstone; 1999:2223–2253.
69. Athanasian EA. Principles of diagnosis and management of musculoskeletal tumors. In: Green DP, Hotchkiss RN, Pederson WC, eds. Green’s Operative Hand Surgery. 4th ed. New York, NY: Churchill Livingstone; 1999:2206–2222.
70. TerKonda SP, Perdikis G. Non-melanotic skin tumors of the upper extremity. Hand Clin. 2004;20:293–301.
71. Bordalo-Rodrigues M, Rosenberg ZS. MR imaging of entrapment neuropathies at the elbow. Magn Reson Imaging Clin N Am. 2004;12:265–279.
72. Tremble TE, Diao E, Abrams RA, et al. Single-portal endoscopic carpal tunnel release compared with open release: A prospective randomized trial. JBJS (American). 84:1107–1115.
73. Szabo RM. Entrapment and compression neuropathies. In: Green DP, Hotchkiss RN, Pederson WC, eds. Green’s Operative Hand Surgery. 4th ed. New York, NY: Churchill Livingstone; 1999:1404–1447.
74. Disa JJ, Chung KC, Gellad FE, et al. Efficacy of magnetic resonance angiography in the evaluation of vascular malformations of the hand. Plast Reconstr Surg. 1997;99:136–144.
Wrist ligaments and the triangular fibrocartilage complex (TFCC) play an important role in maintaining function, alignment, and stability of the wrist. Recent developments in MRI technologies now permit detailed observation of these structures. In this Chapter, the intrinsic and extrinsic wrist ligaments and the TFCC are discussed. The use of high-resolution MRI as well as MR arthrography is also addressed.
Intrinsic Wrist Ligaments (Intercarpal Ligaments)
The intrinsic ligaments of the wrist form a complex structure that is crucial to wrist stability. The two most clinically important intrinsic wrist ligaments are the lunotriquetral ligament (LTL) and the scapholunate ligament (SLL), which are vulnerable to attritional wear (1). With loss of SLL integrity, the scaphoid tends to flex, whereas the lunate and triquetrum tend to extend, imparting a dorsiflexed intercalated segment instability (DISI) orientation. Conversely, with loss of LTL integrity, the triquetrum tends to extend, whereas the scaphoid and lunate attempt to flex. However, a complete LTL tear is not sufficient to cause static carpal collapse into a volar intercalated segment instability (VISI) orientation (2). VISI-pattern lunotriquetral instability requires associated capsular damage, typically to the dorsal radiocarpal and dorsal intercarpal ligaments.
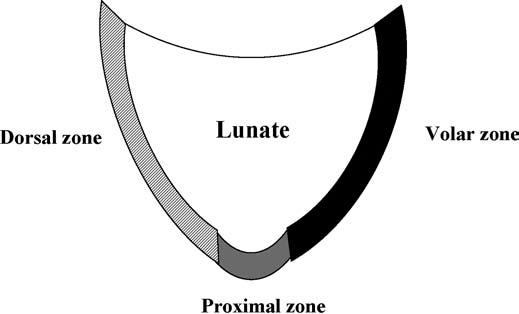
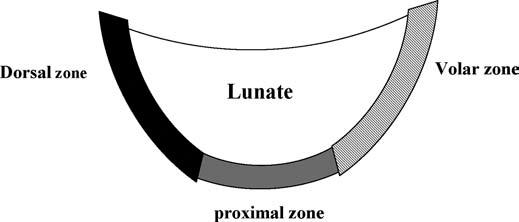
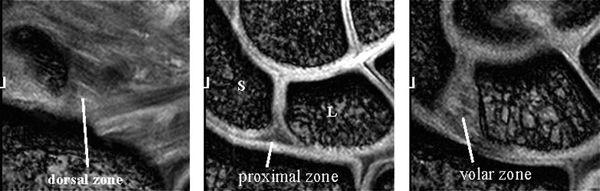
The LTL is V-shaped on sagittal section and has three separate zones: dorsal, proximal, and volar (Fig. 15.1). The dorsal and volar zones are ligamentous. The former is highly important functionally, particularly as a restraint to rotation, whereas the latter is the strongest and thickest of all three zones and transits the extension moment of the triquetrum (2, 3). The proximal zone is fibrocartilaginous and is similar histologically to the TFCC. It is thin (1–1.5 mm or less) and difficult to see reliably (3).
The radiographic appearance of wrists with LTL tears is often normal. Lunotriquetral dissociation results in disruption of the smooth arcs that are formed by the proximal and distal joint surfaces of the proximal carpal row (Gilula’s arcs 1 and 2) and the proximal joint surfaces of the distal carpal row (Gilula’s arc 3). Arthrography can be valuable in demonstrating leakage or pooling of the contrast medium at the lunotriquetral interspace. On arthrography of the normal wrist, however, age-related perforations of the LTL proximal zone, other communications between the radiocarpal and midcarpal joints, and asymptomatic LTL tears have been reported. Therefore, the results of arthrography must be correlated with clinical examination findings (2).
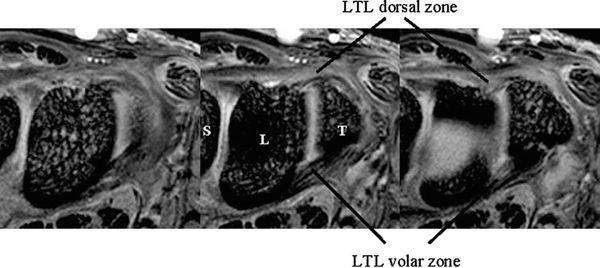
The dorsal and volar portions of the LTL are biomechanically more important than the proximal portion of the LTL. Therefore, the axial plane may be better for assessing LTL injuries, although many previous MR studies have discussed LTL injuries in the coronal plane. With injury of the dorsal and volar portion, a more clinically useful approach would be high-resolution MRI in the axial plane using a microscopy coil (4) (Fig. 15.2).
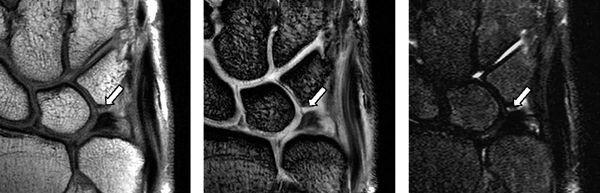
Complete tears of the LTL and SLL have been classified on MRI by either abnormal ligament morphology (to the point of absence of the entire ligament) or by the presence of fluid through the entire ligament on T2-weighted images (5). Partial tears of the LTL and SLL have been diagnosed by two basic criteria: the presence of fluid through a portion of the ligament and partially altered ligament morphology visualized on any or all sequences (6) (Figs. 15.3 and 15.4). However, the sensitivity for diagnosing partial intercarpal ligament tears, particularly those of the LTL, is limited.
Accurate diagnosis of LTL injury with MRI is often difficult because of low resolution and low contrast. The accuracy of MRI in making a diagnosis of LTL tear has been disappointing with sensitivities from 0% to 56% and specificities from 46% to 100% (5, 7, 8). In addition, a potential cause for false-positive MR findings of LTL tear is lack of familiarity with the normal MR appearance at the ligament–bone interface (9). Smith and Snearly (9) reported that the proximal zone of the LTL showed a variety of shapes and signal intensities. Yoshioka et al. (10) also recently reported the shape and signal intensity of the proximal zone of the LTL with high-resolution MRI; they concluded that gaining familiarity with the normal MR appearance of the LTL proximal zone using a high-resolution technique is important to improve diagnosis of LTL injury (Fig. 15.5). The LTL proximal zone has a triangle shape in more than 85% of subjects on gradient recalled echo images. The linear intermediate or high signal intensity traversing the distal surface, or both distal and proximal surfaces, of the proximal LTL is seen in approximately two thirds of subjects (10). The amorphous shape may represent diffuse degenerative changes (9). The morphologic and signal intensity characteristics of the LTL interface with the lunate and triquetral bones vary (9).
FIGURE 15.1 Diagram of the lunotriquetral ligament (LTL) on sagittal section. The V-shaped LTL has three separate zones: dorsal, proximal, and volar. The dorsal and volar zones are ligamentous, whereas the proximal zone is fibrocartilaginous.
FIGURE 15.2 High-resolution MRI of the lunotriquetral ligament in the axial plane obtained with a microscopy coil (Philips Inc., Eindhoven, The Netherlands). Normal band-like structure of the dorsal and volar zones is well appreciated in this plane.
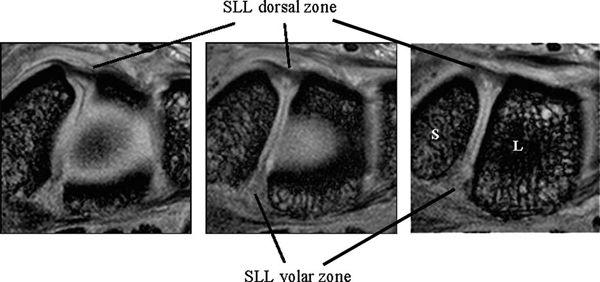
The SLL has three zones and appears horseshoe-shaped or C-shaped, unlike the V-shaped LTL (Fig. 15.6). The SLL is approximately 18 mm long and 2 to 3 mm thick (3). In the dorsal aspect, the SLL forms a strong ligamentous zone that appears to provide most of the ligament’s resistance to diastasis between the proximal poles of the scaphoid and lunate (11). In the proximal zone, the SLL is fibrocartilaginous like the LTL; here, the SLL proximal zone is weakest and can undergo degenerative perforation. The volar zone of the SLL is ligamentous but thinner and is separated by loose vascular connective tissue, which causes it to be less easily observed on conventional MRI. On axial images, the ligamentous dorsal zone appears thick and homogeneous with low signal intensity, whereas the volar zone shows an inhomogeneous striated structure (Fig. 15.7). On coronal images, the dorsal zone of the SLL clearly shows a striated pattern. The striated band-like structure of the volar zone of the SLL is observed only with high-resolution imaging (Fig. 15.8). On high-resolution MRI, the shape of the SLL proximal zone varies widely from slice to slice, unlike the LTL, which keeps a relatively similar triangular shape. Totterman and Miller (12) classified the SLL’s proximal zone into three patterns: a band-like dorsal portion, a triangular or flat triangular middle portion, and a trapezoidal volar portion. The proximal zones of the SLL and LTL insert directly on the hyaline cartilage of the scaphoid, lunate, and triquetral bones, whereas the dorsal and volar components often insert directly on the carpal bones (13).
FIGURE 15.3 LTL injury on proton density-weighted image (PDWI), gradient recalled echo (GRE) image, and short tau inversion recovery (STIR) image from the left. The MR images show the absence of the lunotriquetral ligament proximal zone (arrows).
FIGURE 15.4 Scapholunate ligament (SLL) injury on proton density-weighted image (left) and T2*-weighted image (right). The MR images show discontinuity of the SLL and fluid intensity between the scaphoid and the lunate.
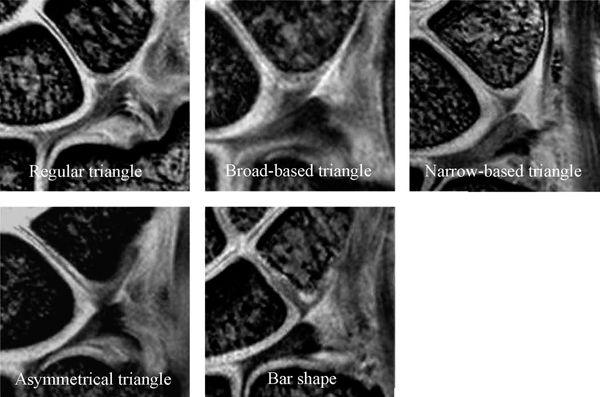
FIGURE 15.5 The lunotriquetral ligament (LTL) shape of the proximal zone with high-resolution coronal gradient recalled echo images. The shape of the LTL proximal zone can be classified into five types (Type 1: regular triangle, Type 2: broad-based triangle, Type 3: narrow-based triangle, Type 4: asymmetric triangle, and Type 5: bar shape).
FIGURE 15.6 Diagram of the scapholunate ligament (SLL) on sagittal section. The C-shaped SLL has three separate zones: dorsal, proximal, and volar. The dorsal and volar zones are ligamentous and the proximal zone is fibrocartilaginous similar to those of the lunotriquetral ligament.
FIGURE 15.7 High-resolution MRI of the scapholunate ligament in the axial plane. The ligamentous dorsal zone appears thick and homogeneous with low signal intensity, whereas the volar zone shows an inhomogeneous striate structure.
FIGURE 15.8 High-resolution MRI of the scapholunate ligament (SLL) in the coronal plane. The dorsal and volar zones of the SLL show a striated pattern, whereas the SLL proximal zone shows a triangle shape.
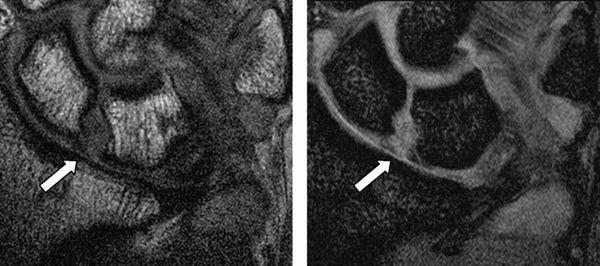
FIGURE 15.9 T1-weighted fat-suppressed image from an MR arthrogram shows a partial tear of the scapholunate ligament (arrow). No high-signal–intensity contrast extends from the radiocarpal region to the midcarpal compartment, indicating a partial tear.
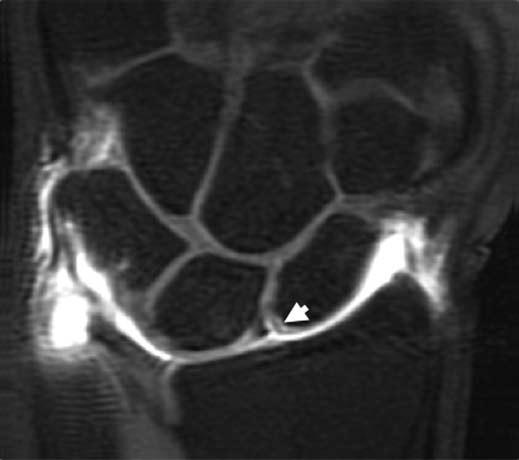
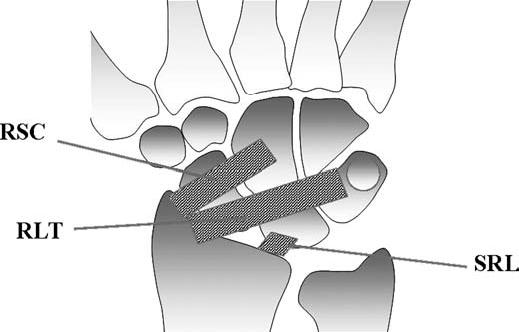
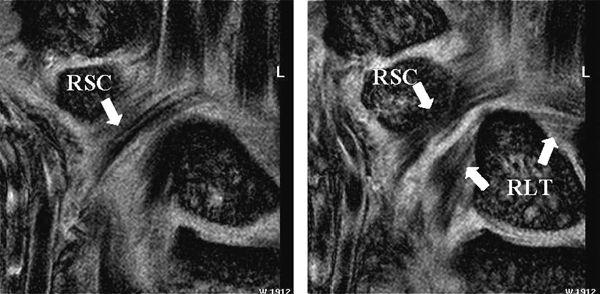
FIGURE 15.10 Diagram of the volar extrinsic wrist ligaments. At the volar aspect, there are three major extrinsic ligaments: the radioscaphocapitate (RSC), radiolunotriquetral (RLT), and short radiolunate (SRL) ligaments.
Conventional radiography is not the appropriate imaging modality for assessing possible lesions of the SLL and can diagnose scapholunate instability only in cases with an enlarged scapholunate gap or abnormal carpal angles (DISI). Direct arthroscopy can detect ligamentous disruptions by demonstrating communication between the radiocarpal and midcarpal joints, but controversy exists concerning its ability to differentiate between asymptomatic degenerative tears and symptomatic acute or chronic lesions (14).
The possible reasons for the suboptimal diagnostic accuracy of MRI for the diagnosis of SLL tears are similar to those for LTL tears: limitations in spatial resolution, limited signal-to-noise ratios with use of available receiver coils, inherent limitations in contrast between ligamentous structures, and unfamiliarity with the normal range of SLL appearance on MRI (15).
Three-compartment MR wrist arthrography has several advantages over nonenhanced MRI of the wrist in diagnosing SLL and LTL injuries. First, the joint cavities of the wrist can be fully expanded so that most of the ligamentous and capsular structures are stretched and can be directly visualized and evaluated (16). Second, the high signal of gadolinium–diethylene–triamine–penta-acetic acid leads to excellent delineation of all parts of these ligaments, which show low signal intensity. Third, MR arthrography can precisely localize and quantify SLL or LTL leaks, which have a significant impact on diagnosis and treatment (Fig. 15.9) (16). Limitations of MR arthrography compared with nonenhanced MRI are the additional cost and invasiveness of MR arthrography and the need to inject contrast medium into the joint. The technique is fairly complicated and time-consuming (16). Scheck et al. (16) reported that with wrist arthroscopy as the standard of reference, sensitivity and specificity values for SLL perforations were 52% and 34%, respectively, for nonenhanced MRI and 90% and 87%, respectively, for MR arthrography. They concluded MR arthrography, using three-dimensional volume acquisition with thin slices, shows the precise location and magnitude of ligamentous defects of all parts of the SLL, correlates well with wrist arthroscopy, and has potential implications for diagnosis and treatment planning (16).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree