
Diagnosis: Glioblastoma multiforme
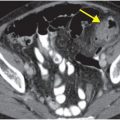
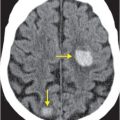
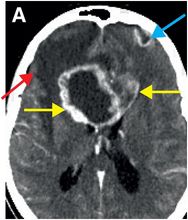
Contrast-enhanced CT (A) reveals an irregular, peripherally enhancing, centrally necrotic transcallosal mass (yellow arrows). Peritumoral low attenuation within the right frontal white matter (red arrow) represents a combination of infiltrative tumor and vasogenic edema. A second peripherally enhancing lesion is seen within the anterior left frontal lobe (blue arrows), consistent with multicentric disease.
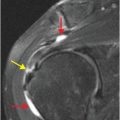
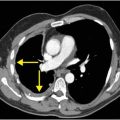
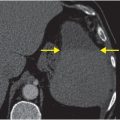
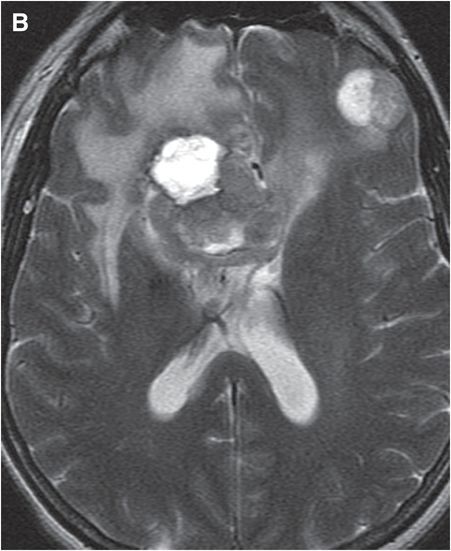
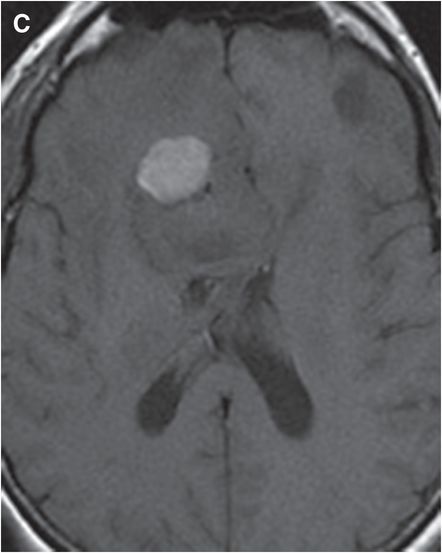
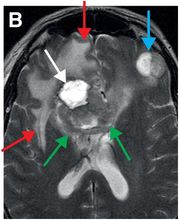
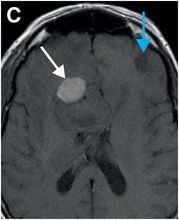
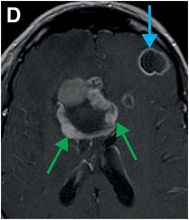
Axial T2-weighted (B), T1-weighted (C) and T1-weighted post-gadolinium (D) MR sequences show multiple enhancing foci, some with high signal on T1- and T2-weighted imaging (white arrows) due to hemorrhagic or proteinaceous contents. Hypercellular components (green arrows) are hypointense on T2-weighted imaging and enhance after administration of gadolinium. Right greater than left nonenhancing white matter abnormality (red arrows) represents combination of vasogenic edema and tumor infiltration.
Discussion
Overview of glioblastoma multiforme
Glioblastoma multiforme (GBM, WHO grade IV glioma) is the most common aggressive primary brain tumor, comprising about 12–15% of all intracranial tumors and 50–60% of all glial tumors. The incidence of GBM is approximately 2–3 per 100,000 people per year. GBM can be primary, arising de novo (60%), or secondary (40%), developing from pre-existing low grade (WHO Grade II) or anaplastic (WHO Grade III) astrocytomas. These are thought to represent distinct disease processes that evolve via different genetic pathways. The average age in primary GBM is older (62 years) than in secondary GBM (45 years). Treatment response tends to be poorer in primary GBM. GBM occurs slightly more often in Caucasians, with a male-to-female ratio of 3:2.
GBM is an infiltrative tumor that travels along white matter tracts. GBM may be focal or multicentric, with half involving more than one lobe or both hemispheres. Approximately 2–7% truly arise in multiple independent sites, with the rest of apparently “multicentric” disease representing diffuse infiltration. Temporal lobe involvement is the most common, followed closely by the parietal and frontal lobes. Occipital lobe disease is the least common. Occasionally, tumor extends to the ventricular system, leading to CSF spread with leptomeningeal disease and drop metastases.
Clinical presentation of glioblastoma
Pathology of glioblastoma
Histologically, GBM is differentiated from lower-grade gliomas by necrotic tissue surrounded by poorly differentiated pleomorphic astrocytes and microvascular proliferation.
Grossly, GBM is poorly circumscribed, with central yellowish tissue from myelin breakdown amid blood products of varying age.
Glioblastomas are also distinguished from other gliomas by greater genetic alterations, with mutations in genes such as p53 and EGFR. Extracranial GBM metastasis is rare, though has been described.
Gliosarcoma represents approximately 2.1% of GBM and is histologically distinguished by both glial and sarcomatous components. Gliosarcoma also has a greater propensity for extracranial metastasis.
Imaging of glioblastoma
Heterogeneous imaging appearances of GBM reflect the complexity of its molecular and histological features. MR is superior to CT in evaluating GBM, especially in the detection of leptomeningeal disease.
The spectrum of imaging findings ranges from small and homogeneously enhancing foci to large and heterogeneously enhancing masses.
The classic imaging appearance is a necrotic “butterfly” lesion straddling the corpus callosum to involve both cerebral hemispheres.
T2 prolongation surrounding tumor represents a combination of vasogenic edema and tumor infiltration. This is in contrast to brain metastases, where the abnormal T2 prolongation usually reflects vasogenic edema without tumor cells. It is important to note that GBM is known to extend microscopically beyond the margins of signal abnormality seen on MR.
Post-treatment imaging of glioblastoma
Differentiating tumor from post-treatment changes can be difficult. Modalities such as MR perfusion (elevated cerebral blood volume and vascular permeability due to tumoral neovascularity), PET (increased metabolism in tumor) and MR spectroscopy (elevated choline and lactate and decreased N-acetyl aspartate [NAA] peaks) are generally reserved for follow-up assessment of treatment response and tumor recurrence.
Prognosis and treatment of glioblastoma
Prognosis for patients with GBM remains very poor despite therapeutic and diagnostic advances; median survival is approximately 3 months without treatment and 12 months with treatment. Fewer than 25% of patients survive at 2 years and fewer than 10% survive by 5 years. Slightly more favorable prognosis is conferred by age less than 50 at presentation, higher Karnofsky score (measure of ability for daily tasks in cancer patients), and at least 98% surgical tumor resection.
Treatment goals for GBM are aimed primarily at prolongation of survival and palliation rather than at cure. Initial treatment strategies combine surgery, radiation, and chemotherapy (mainly temozolomide, which sensitizes tumor cells to radiation). Bevacizumab (an anti-angiogenic agent) is generally reserved for progression or recurrence.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree