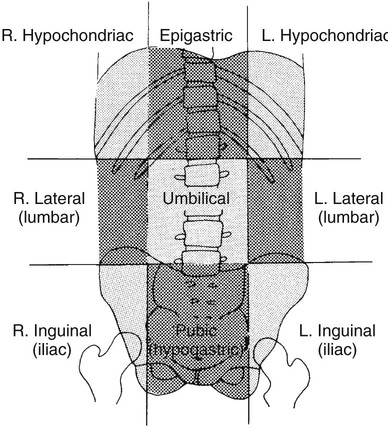
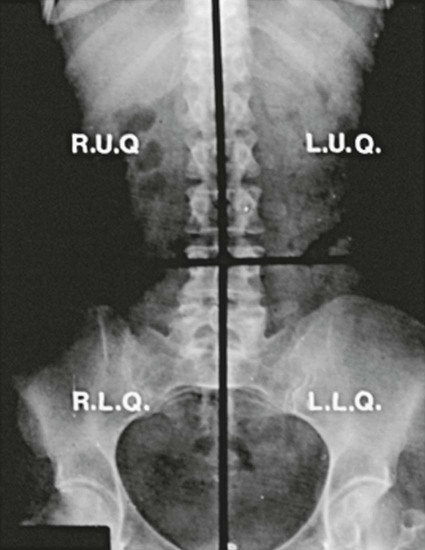
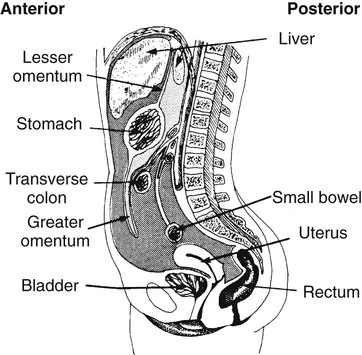
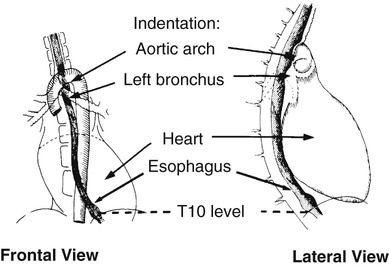
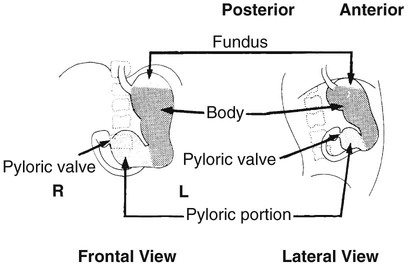
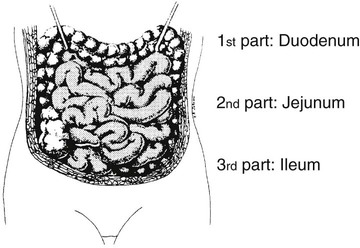
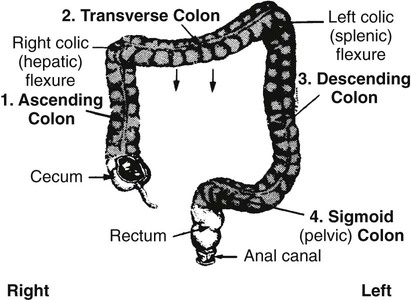
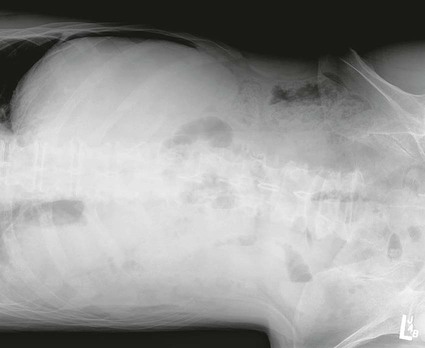
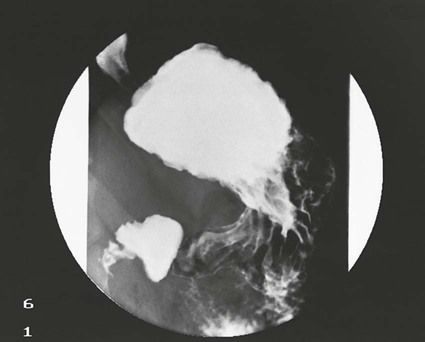
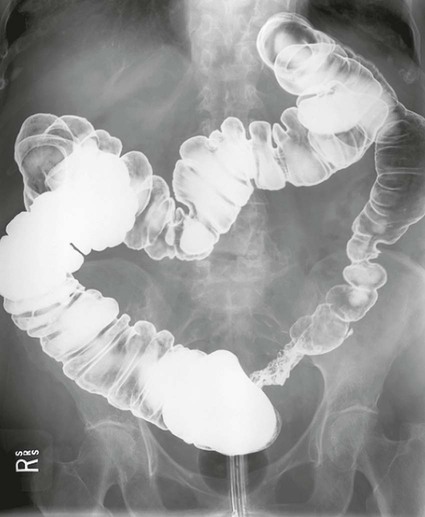
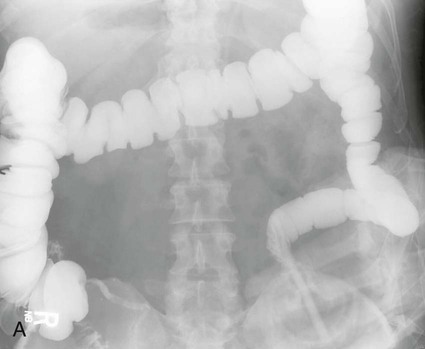
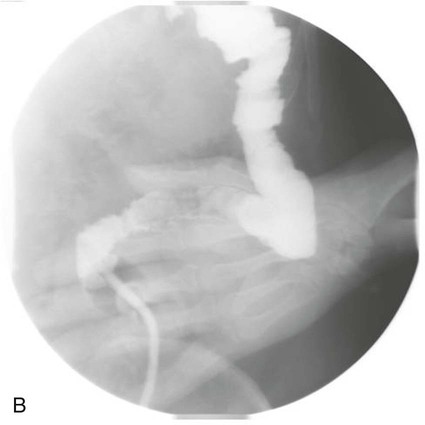
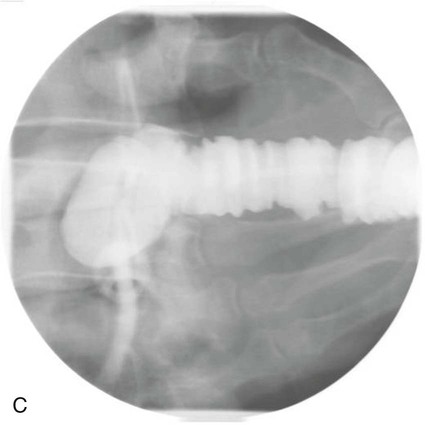
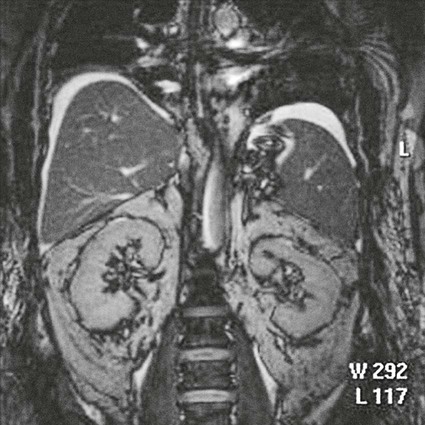
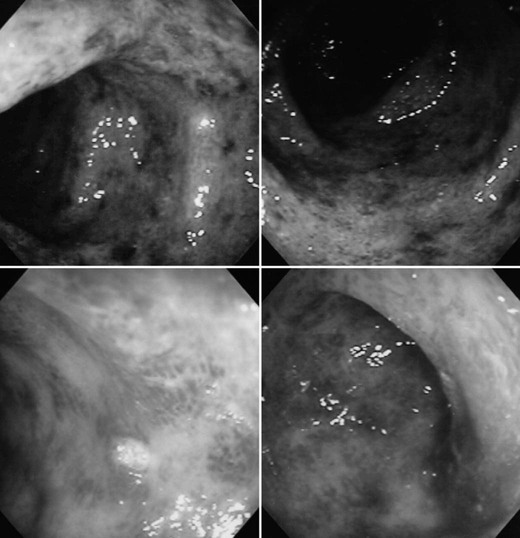
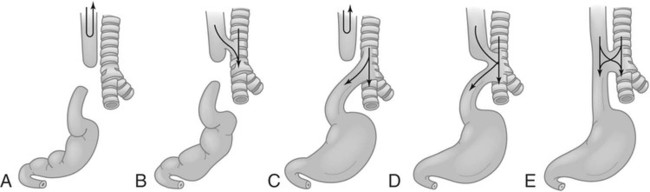
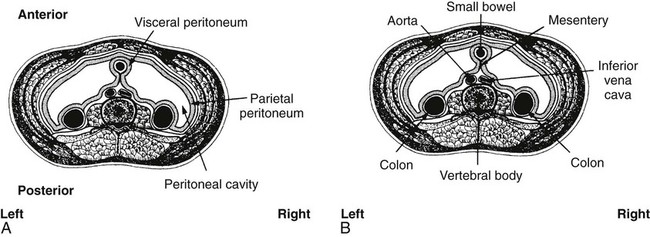
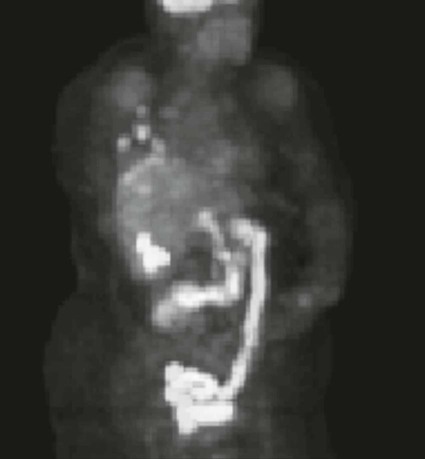
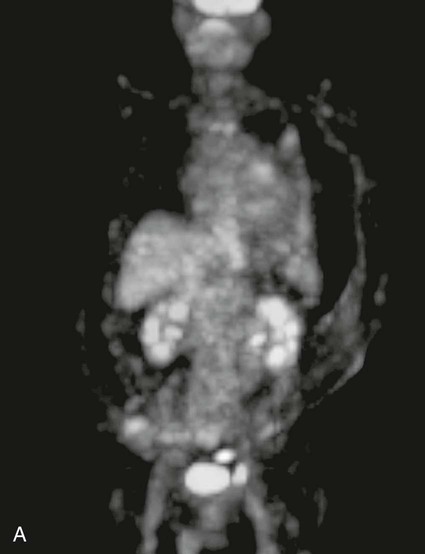
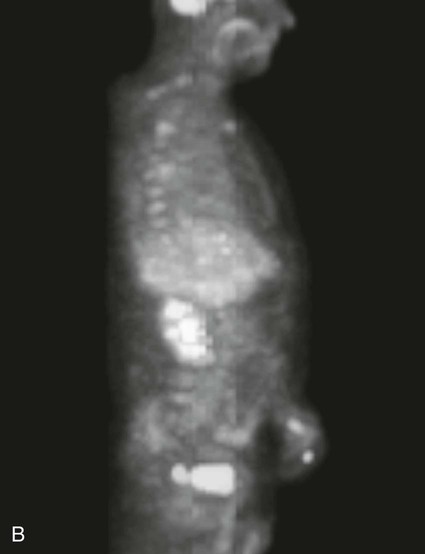
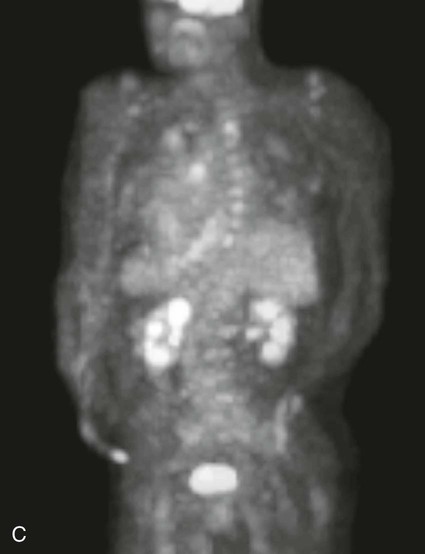
On completion of Chapter 5, the reader should be able to: • Describe the anatomic components of the abdomen and the gastrointestinal system and how they are visualized radiographically. • Compare and contrast the various imaging modalities used in the evaluation of the abdomen and its contents. • Identify the tubes and catheters related to the gastrointestinal system by type, and briefly explain their use. • Characterize a given condition as congenital, inflammatory, neurogenic, or neoplastic. • Identify the pathogenesis of the gastrointestinal pathologies cited and typical treatments for them. • Describe, in general, the radiographic appearance of each of the given pathologies. The abdomen is composed of the abdominal and pelvic cavities and is often divided into nine anatomic regions: (1) right hypochondriac, (2) epigastric, (3) left hypochondriac, (4) right lumbar, (5) umbilical, (6) left lumbar, (7) right iliac, (8) hypogastric, and (9) left iliac (Fig. 5-1). It may also be described in terms of quadrants: right-upper (RUQ), right-lower (RLQ), left-upper (LUQ), and left-lower (LLQ) (Fig. 5-2). The abdominal cavity contains organs of the digestive system (stomach and intestines), the hepatobiliary system (liver, gallbladder, and pancreas), the urinary system (kidneys and ureters), and the circulatory system (spleen). The pelvic cavity contains the bladder, portions of the intestines, and the reproductive organs. The abdominal cavity is lined by the peritoneum, a serous membrane (Fig. 5-3, A). The serous lining attached to the abdominal organs is the visceral peritoneum. The peritoneum attached directly to the abdominal wall is the parietal peritoneum. The mesentery is a double fold of parietal peritoneum projecting from the posterior abdominal wall in the lumbar region (see Fig. 5-3, B). Most of the small bowel is attached to the outer edge of the mesentery. The greater omentum is a double fold of peritoneum that attaches to the duodenum, stomach, and transverse colon. It hangs loosely over the intestines. The lesser omentum is a fold of peritoneum that attaches the liver to the lesser curvature of the stomach and the duodenum (Fig. 5-4). The esophagus is the first part of the GI system. It is approximately 10 to 12 inches long and extends from the posterior pharynx to the stomach (Fig. 5-5). The upper esophagus is midline, but it courses to the left to pass behind the aortic arch, which indents the esophagus. Other indentations occur at the level of the left main stem bronchus and at the gastroesophageal junction. As it passes downward, the esophagus follows the curvature of the thoracic spine and thoracic descending aorta. The stomach occupies the body’s LUQ, with the cardiac orifice at the level of the tenth or eleventh thoracic vertebra and the pyloric canal just to the right of the first or second lumbar vertebra (Fig. 5-6). Peristalsis churns the gastric content and propels it toward the pylorus. Gastric emptying of liquids is accounted for by the peristalsis initiated in the fundus of the stomach; gastric emptying of solids requires a to-and-fro action of the antrum and pylorus. In the presence of masses, inflammation, or diabetes, the peristaltic activity may be diminished. When the stomach is filled with barium, its curvatures are visible as generally smooth contours. The rugae appear as longitudinal ridges within the stomach. The small bowel includes the duodenum, jejunum, and ileum. It arises from the stomach at the duodenal bulb and courses to the ileocecal valve (Fig. 5-7), over a length of nearly 21 feet. The duodenal C-loop moves posteriorly from the gastric antrum to its ending at the ligament of Treitz. The jejunum begins here and coils in the LUQ before terminating at the ileum in the RUQ. The ileum then courses through the RLQ and LLQ to terminate at the ileocecal junction. When filled with barium the segments of the small bowel are distinguishable by their appearance. The duodenal mucosa is indicated by its transverse rigid appearance. The jejunal mucosa appears delicate and feathery. Ileal folds look like those of the duodenum, although not as large. The large bowel extends from the terminal ileum to the anus for a length of about 6 feet (Fig. 5-8). Its distinct regions are the cecum, orifices for the terminal ileum and the appendix, ascending colon and hepatic flexure, transverse colon and splenic flexure, descending colon, sigmoid, rectum, and anus. The cecum is usually retroperitoneal and anterior and lies against the abdominal wall. The ascending colon is also retroperitoneal and becomes more posterior as it ascends to lie adjacent to the undersurface of the liver. The hepatic flexure, transverse colon, and splenic flexure are all intraperitoneal. They lie more anteriorly and are attached to the posterior abdominal wall by the mesocolon, a double layer of peritoneum. The descending colon is retroperitoneal, moving posteriorly as it descends. The peritoneal sigmoid colon lies in the pelvis and is quite mobile. Structures located anterior to it include the bladder and, in women, the uterus. Posterior structures include the iliac arteries and sacral nerves. The rectum is extraperitoneal, beginning at the third sacral segment and following the sacrococcygeal curve to the anus. The valves of Houston are prominent transverse folds in the distal rectum, where it dilates. The anus forms the distal 1 to 2 inches of the large bowel and contains no peritoneal covering. In a normal abdomen (Fig. 5-9), varying amounts of gas and fecal material are always present in an unprepared patient. The liver, kidney, spleen, and psoas muscle shadows are variably outlined because of the lucent layer of fat surrounding them. Properitoneal fat stripes are visible as radiolucencies extending laterally from the costal margins down to the iliac crests. The aorta and pancreas are not normally seen unless they are calcified, as might be expected in an older patient in the case of the aorta or in a patient with chronic calcific pancreatitis. The inferior margin of the liver should lie at or above the level of the right twelfth rib. The left kidney is usually slightly higher than the right kidney because of the presence of the liver superior to it. The top of the left kidney generally lies at the vertebral level of T11-T12, whereas the top of the right kidney is approximately 1 cm lower in position. In terms of renal size, the kidneys are generally the length of three vertebrae in children over 1 year of age. By adulthood, the kidneys reach the length of approximately two and a half vertebrae. Few, if any, air–fluid levels are present in the normal patient who is radiographed in the erect position. Limited fluid levels in the small bowel and large bowel, however, may be considered normal. Fluid levels are abnormal when they are seen in dilated bowel loops or when they are numerous. The intestinal gas pattern may be confusing to the diagnostician. In infants and children, gas may be scattered throughout the bowel, but in adults gas is normally seen only in the stomach and colon. Small bowel gas in an adult, therefore, may indicate a pathologic process. In some patients, gas may be recognizable only on erect radiographs because of the presence of intraluminal fluid. Free air should not be visible in the peritoneal cavity and is indicative of a bowel perforation or other pathologic entities that introduce air into the peritoneum. Erect abdominal radiographs must include the diaphragm to assess for free air, and in instances in which the patient is unable to stand, a left lateral decubitus abdomen should be obtained (Fig. 5-10). A series of images are obtained during or after fluoroscopy, with the projections differing from one institution to another. Typical patient positions include recumbent posteroanterior (PA) projection to demonstrate the entire stomach and duodenal bulb, right anterior oblique (RAO) to highlight the pyloric canal and duodenal bulb (Fig. 5-11), right lateral to show the duodenal bulb, duodenal loop, and retrogastric space, and left posterior oblique (LPO) to demonstrate the gastric fundus. Proper positioning relates significantly to the patient’s body habitus. Generally, the more hypersthenic a patient is, the higher and more transverse the stomach tends to lie. For other body habitus (i.e., sthenic, hyposthenic, and asthenic), the stomach is more J-shaped, lying lower and closer to the spine. In some instances, the barium sulfate mixture may be followed as it progresses through the small intestines. Radiographs are exposed at set intervals to determine GI motility and to demonstrate abnormalities within the small bowel. Once the contrast agent reaches the ileocecal valve, the small bowel study is complete, typically within 2 to 3 hours (Fig. 5-12). The lower GI tract is examined by administering a barium enema through the rectum. This examination demonstrates abnormalities of the large bowel and intraluminal neoplasms. The barium enema is performed in a single-contrast fashion with only barium or as a double-contrast study using barium sulfate in combination with a negative contrast agent (e.g., air). The negative contrast agent distends the lumen, allowing improved visualization of the mucosal lining (Fig. 5-13), especially small polyps and intraluminal tumors. In either case, the radiologist typically obtains a series of images during fluoroscopy, with the patient in various positions to highlight certain areas of the colon (e.g., flexures). The radiographer may also expose a series of radiographs of the contrast-enhanced large bowel per the radiologist’s instructions (Fig. 5-14). After evacuation of the barium sulfate mixture, the radiographer will obtain a “postevacuation” radiograph to visualize colon contraction and to demonstrate the mucosa. If a patient has had a surgical enterostomy procedure, the contrast agent may be administered through the opening in the abdominal wall to the specific area of the GI system (Fig. 5-15). Special care needs to be taken when imaging the patient with some types of stoma because this area can be particularly sensitive. The role of magnetic resonance imaging (MRI) in the abdomen has expanded as a result of faster sequences and shorter scan times. Evaluation of the GI tract is still limited by bowel motion; however, MRI is useful in demonstrating the presence of retroperitoneal masses impinging on the GI system. Breath-hold imaging in MRI allows the technologist to visualize abdominal organs in a matter of seconds (Fig. 5-16). A few different imaging sequences are used to differentiate between normal tissue and pathology. The most common of the imaging sequences are T1-weighted images for optimal visualization of anatomic structures and T2-weighted images for optimal visualization of diseased tissues. Three-dimensional contrast-enhanced magnetic resonance angiography (MRA) is also used in imaging of the arterial vessels of the abdomen. Gastric emptying scans are used to assess the rate food exits the stomach into the duodenum. The examination is performed by having the patient consume either a solid or liquid radiolabeled meal and then observed via a gamma camera as it passes out of the stomach. Typically, a high level of radioactivity maintained within the stomach after an extended period would indicate poor gastric emptying (Fig. 5-17). In addition, the test may also be used to monitor the response of promotility drug treatments such as that of metoclopramide or erythromycin. One drawback of the test is that it cannot differentiate between physical obstruction and gastroparesis; that is, further diagnostic studies will often need to be ordered if the patient exhibits a delay in gastric emptying. Positron emission tomography (PET) may be used to evaluate and stage GI cancers (Fig. 5-18). PET has been proven to demonstrate approximately 20% of esophageal cancerous lesions (Fig. 5-19) undetected by CT. As noted earlier, endoscopy is the use of tubular fiberoptic devices to look inside the GI tract and other hollow organs or cavities of the body. As its sophistication and specificity have increased, it is assuming a greater role in diagnosis and therapy of the GI tract. Upper endoscopy is capable of seeing down into the esophagus, stomach, duodenum (including the ampulla of Vater), and even the proximal jejunum. Colonoscopy allows retrograde visualization of the rectum as far as the terminal ileum. The small bowel is still largely out of reach through endoscopy; however, the advent of capsule endoscopy, which involves the ingestion of a small camera pill, has resulted in its increased use in the diagnosis of small bowel tumors. The capsule encases a digital camera that transmits images to a recording device worn on a belt. The capsule has a gastric transit time of approximately 1 hour and small-bowel transit time of 3 to 4 hours. This technology is indicated in diagnosis of obscure GI bleeding, which is often caused by a lesion. This technology, however, has several drawbacks such as inability to identify the precise location of pathology, inability to biopsy or treat located pathology, and lack of reimbursement by insurance companies. The only contraindication to performing capsule endoscopy is a bowel obstruction. Photographic views of the interior of the body provide readily diagnosable information (Figs. 5-20 and 5-21). Therapeutic applications of endoscopy are numerous. They include polyp removal, injection and thermal methods to stop hemorrhaging, sclerosing and banding of esophageal varices, lesion biopsy, sealing of tracheoesophageal fistulas, stone removal, esophageal prosthesis insertion, and laser tumor removal (both generally for palliative purposes). In addition, enteric wall stents have been used to open the lesions in nonoperative malignancies of the colon. An enteral tube is a small-caliber tube used to deliver a liquid diet directly to the duodenum or jejunum. It most commonly has a weighted end to hold the tube in the proper placement. The Dobhoff tube is a common radiopaque enteral tube (Fig. 5-22). Other common types of prolonged enteral tubes include the Corpak tube and the Entriflex tube. Nasoenteric decompression tubes are used to remove gas and fluids in the prevention and treatment of abdominal distention. At one end, these tubes have a balloon or rubber bag filled with air, mercury, or water to stimulate peristalsis and facilitate passage through the pylorus into the intestinal tract. The Miller-Abbott tube is a common type of double-lumen decompression tube. It is passed through the nose, pharynx, and esophagus with the balloon uninflated. Once the end of the tube reaches the stomach, the balloon is inflated and the tube is pulled back until it stops at the cardiac sphincter. The patient is then placed on his or her right side in the semierect position, and the air is withdrawn from the balloon and replaced with mercury. Progress of the tube is assessed by taking abdominal radiographs at regular intervals. The Harris and Cantor tubes are other types of decompression tubes. Unlike the Miller-Abbott tube, however, the Cantor tube (Fig. 5-23) and the Harris tube contain a single lumen. Atresia is a congenital absence or closure of a normal body orifice or tubular organ. Esophageal atresia is a rare congenital anomaly in which the esophagus fails to develop past some point, resulting in discontinuation of the esophagus (Fig. 5-24). This anomaly is caused by a defect in cell differentiation of the trachea and esophagus during the fourth to sixth week of embryonic development. The symptoms of esophageal atresia are visible soon after birth and include excessive salivation, choking, gagging, dyspnea, and cyanosis. Diagnosis of this congenital anomaly may be established by inability to pass an NG tube into the stomach. If a radiopaque NG tube is used, the terminal end of the pouch may be demonstrated radiographically with a chest radiograph without the use of a contrast agent. Immediate surgery is required to alleviate the problem, and preoperative care must be taken to prevent aspiration pneumonia. The infant may not receive oral feedings, and continuous suction is necessary to prevent aspiration. Under most circumstances, this increased risk of aspiration contraindicates the use of a contrast agent to visualize the extent of the atresia. Usually, a tracheoesophageal fistula is coincident with atresia (Fig. 5-25). This consists of an atresia at the level of the fourth thoracic vertebra with a fistula—an abnormal tubelike passage from one structure to another—to the trachea (Fig. 5-26). In addition, a gastrostomy tube may be placed in the infant’s stomach to prevent reflux of gastric secretions into the trachea through the fistula. Such a condition is incompatible with life for more than 2 to 3 days, but the prognosis is good if the infant is handled appropriately before surgery to prevent aspiration. Duodenal atresia is a congenital anomaly in which the lumen of the duodenum does not exist, resulting in complete obstruction of the GI tract at the duodenum. In some cases, the atresia may be identified before the birth of the infant with the use of sonography. Sonographic evaluation of the fetus should demonstrate a normal stomach with amniotic fluid coursing through it. Atresia is suggested on sonography if a dilated stomach is noted without other fluid collections noted in the fetal abdomen. Although rare (one in approximately 20,000 births), it is evident soon after birth when vomiting begins and the epigastrium becomes distended. A radiographic indication of duodenal atresia is the “double bubble sign.” Gaseous distention of the stomach creates one bubble, and gas in the proximal duodenum creates a second bubble (Fig. 5-27). As with esophageal atresia, oral feedings should be withheld in infants with duodenal atresia. Nasogastric decompression of the stomach is indicated to prevent vomiting and possible aspiration of the gastric contents. Treatment consists of surgery to open the duodenum for connection to the pylorus. During surgery, it is common to examine the other areas of the small bowel and the large bowel for other sites of atresia and malrotation, which often accompany duodenal atresia.
Abdomen and Gastrointestinal System
Anatomy and Physiology
Abdomen
Gastrointestinal System
Imaging Considerations
Radiography
Abdomen.
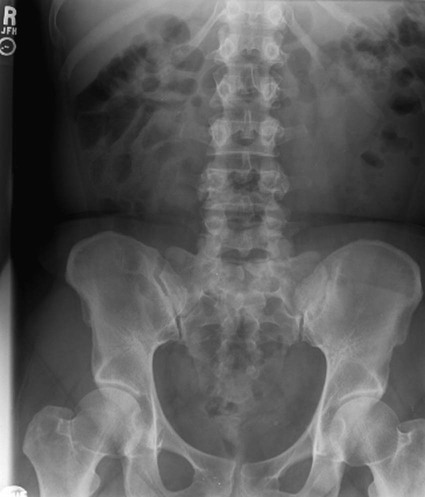
Stomach.
Small Bowel.
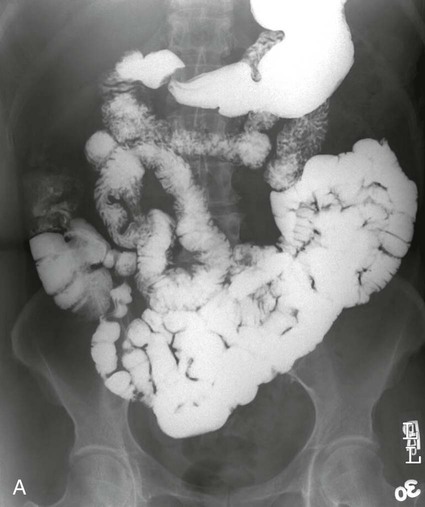
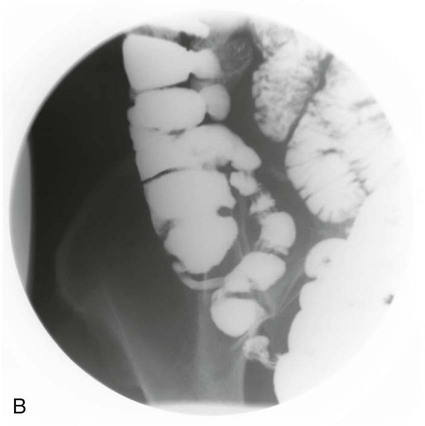
Large Bowel.
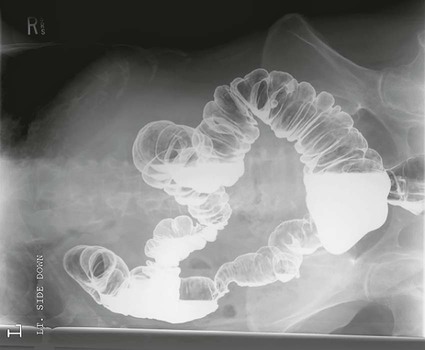
Magnetic Resonance Imaging
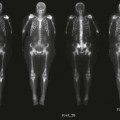
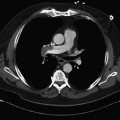
Nuclear Medicine Procedures
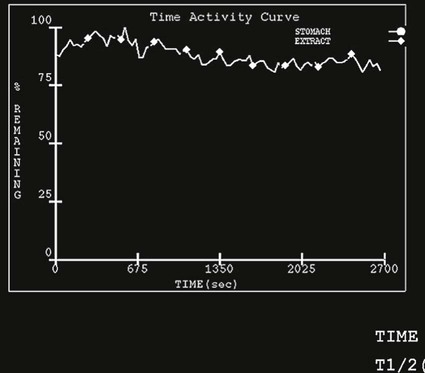
Endoscopic Procedures
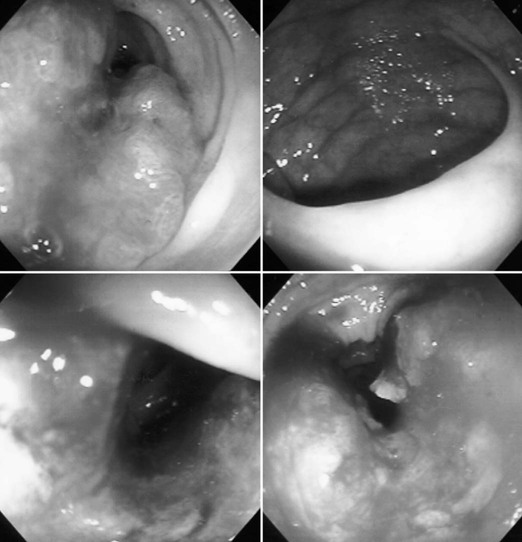
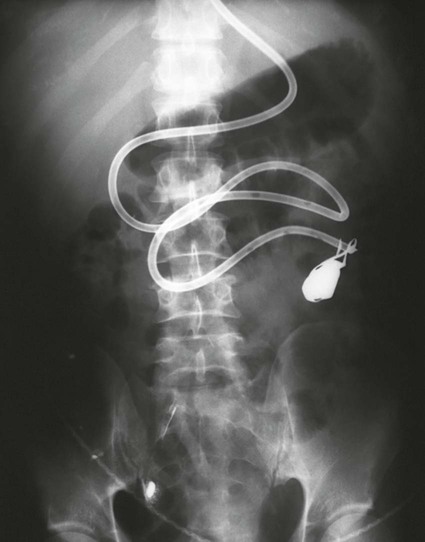
Abdominal Tubes and Catheters
Congenital and Hereditary Anomalies
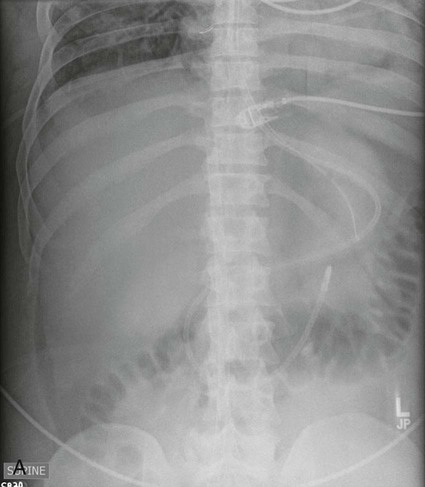
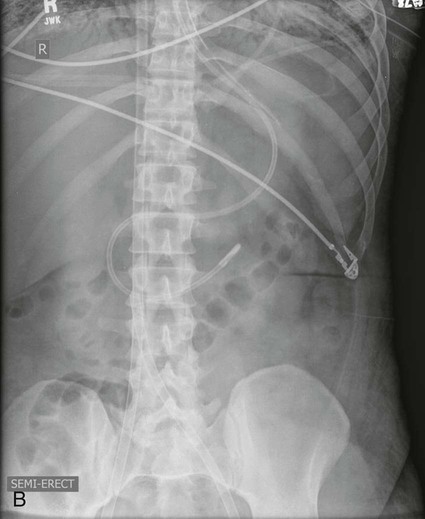
Esophageal Atresia
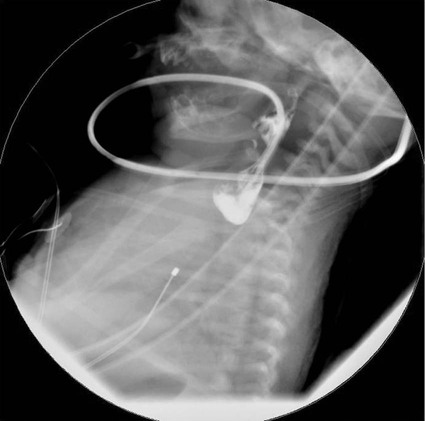
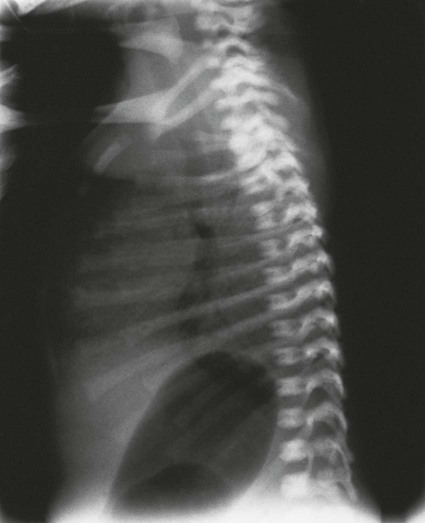
Bowel Atresia
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Abdomen and Gastrointestinal System