Fig. 12.1
Gross Anatomy of the colon
The ascending and transverse colon are part of the midgut (along with the jejunum and ileum) and are supplied by the superior mesenteric artery (Fig. 12.2) [1]. The descending, sigmoid, and rectal portions of the colon form the hindgut and are supplied by the inferior mesenteric artery [1]. For a detailed description of the colonic vascular supply, please refer to Table 12.1.
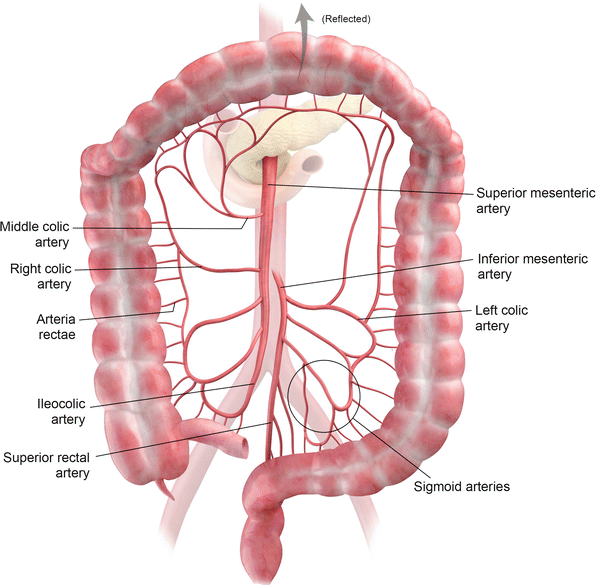

Fig. 12.2
Arterial supply of the colon
Table 12.1
Colonic vascular supply
Colon segment | Arterial supply | Venous drainage |
|---|---|---|
Cecuma | Ileocolic artery | Superior mesenteric vein |
(branch of superior mesenteric artery (SMA)) | ||
Ascendinga | Superior mesenteric vein | |
Inferior portion | Ileocolic artery (branch of SMA) | |
Major portion | Right colic artery (branch of SMA) | |
Near hepatic flexure | Middle colic artery (branch of SMA) | |
Transversea | Middle colic artery (branch of SMA) | Superior mesenteric vein |
Descendinga | Left colic artery (branch of inferior mesenteric artery (IMA)) | Inferior mesenteric vein |
Sigmoida | Inferior mesenteric vein | |
Major portion | Sigmoid artery (branch of IMA) | |
Rectosigmoid junction | Rectosigmoid artery (branch of IMA) | |
Rectum | ||
Superior portion | Rectosigmoid artery (branch of IMA) | Inferior mesenteric vein |
Remainder of the rectum | Superior rectal artery (branch of IMA) | Inferior mesenteric vein |
Middle rectal artery (branch of internal iliac artery) | Internal iliac vein | |
Inferior rectal artery (branch of internal pudendal artery) | Internal iliac vein | |
It is important to note that anastomoses between the left branch of the middle colic artery (off the superior mesenteric artery) and the ascending branch of the left colic artery (off the inferior mesenteric artery) in the region of the splenic flexure of the colon may be poor or absent which makes this colonic segment especially at risk for ischemia [1]. Similarly, the rectosigmoid area (Sudeck’s point) is an additional area at risk for ischemia due to the variability in anastomoses between the rectosigmoid artery and the sigmoid arcade [1]. The marginal artery of Drummond is an important collateral pathway connecting the superior mesenteric artery and the inferior mesenteric artery. The arteria rectae which supply the colon are essentially end arteries.
Lymphatic drainage is generally along vascular pathways with the ascending and transverse colon draining into the superior mesenteric nodes located along the superior mesenteric artery. The left colic nodes (associated with the descending colon and sigmoid colon) drain into the inferior mesenteric nodes as do the superior rectal nodes [1]. By comparison, the middle rectal nodes drain along the internal iliac vessels and the inferior rectal nodes drain along the external iliac vessels [1].
At the histologic level, four discrete layers compose the colonic wall: mucosa, submucosa, muscularis externa, and serosa [2]. The mucosa includes, from the lumen outward, an epithelial layer, a connective tissue layer called the lamina propria, and a smooth muscle layer called the muscularis mucosa [2]. The purpose of the epithelial layer is to resorb water and electrolytes [2]. Numerous goblet cells also secrete mucin into the colonic lumen to facilitate the passage of feces with the proportion of goblet cells increasing toward the rectum [2]. Gut-associated lymphatic tissue is present in the lamina propria. However, lymphatic vessels are not present in the lamina propria, and this absence of lymphatic vessels is thought to account for the slow rate of metastases in some colon cancers [2].
The submucosa is a connective tissue layer. The muscularis externa is composed of two layers of muscle: The outer layer of which is formed by three longitudinal bands (taenia coli) which converge to form a complete outer layer over the appendix and rectum [1, 2]. Contraction of these bands results in the haustral folds of the colon seen at imaging. The serosal layer is composed of a thin serous membrane along with the mesothelium and a thin layer of connective tissue [2].
Embryology
The gut tube forms during weeks 3 and 4 of embryonic development. During the course of its development, the gut tube herniates out of the abdomen and then retracts back into the abdomen during week 10, undergoing a total of 270° of rotation in the counterclockwise direction as viewed from the front [3]. Abnormalities in these rotations result in intestinal malrotation. The term “malrotation” describes a spectrum of abnormalities in which intestinal position differs from normal. For example, the small intestine may be located in the right abdomen with the large intestine in the left abdomen in patients with malrotation (Fig. 12.3). Associated shortened mesenteric attachments and fibrous peritoneal bands may predispose such patients to abdominal pain and volvulus [4].
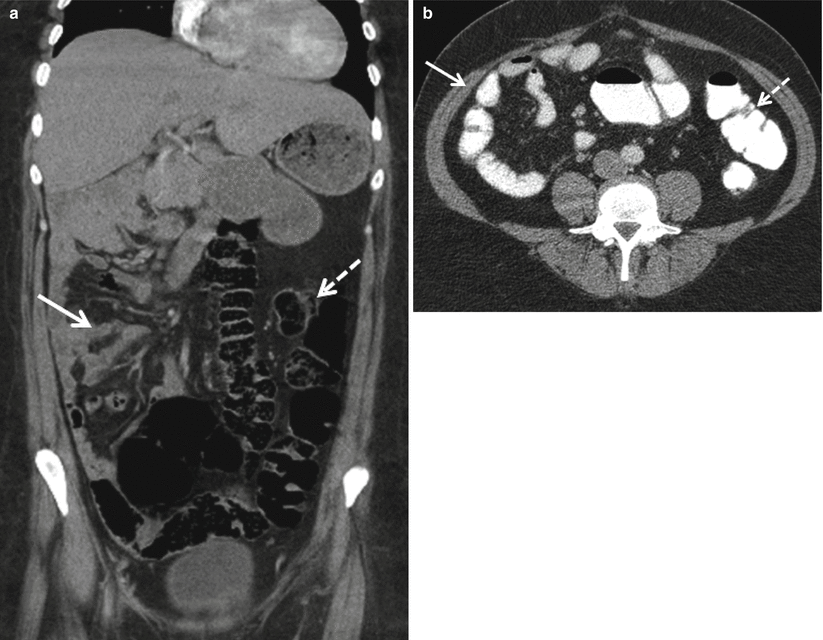

Fig. 12.3
Intestinal malrotation. Axial and coronal nonenhanced CT images from patients with intestinal malrotation. Note the small intestine in the right abdomen (a, b solid arrows) and the colon in the left abdomen (a, b dashed arrows)
By comparison, the term “situs inversus” is used to describe the condition in which internal organ arrangement is the mirror image of the normal anatomy, with the normal anatomy referred to as “situs solitus” [5]. For example, the liver is located in the left abdomen and the cecum is located in the left lower quadrant in patients with situs inversus (Fig. 12.4).
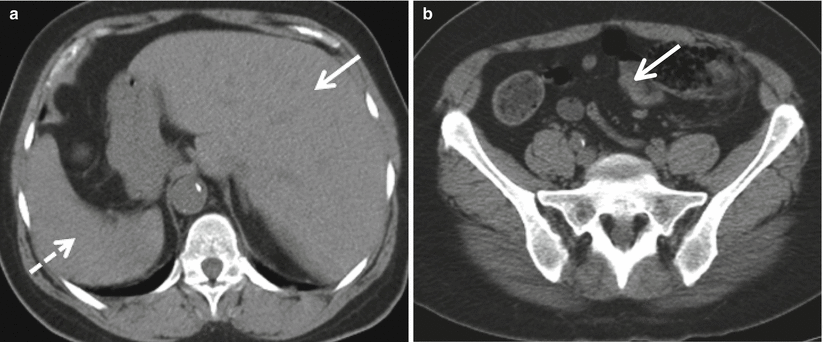

Fig. 12.4
Situs inversus. Axial non-enhanced CT images demonstrate reversed locations of visceral organs with the liver located in the left abdomen (a, solid arrow) and the spleen located in the right abdomen (a, dashed arrow). The ileocecal valve is located in the left lower quadrant (b, arrow)
Though the exact timing is unknown, situs abnormalities are thought to manifest at approximately 28 days of gestation [6]. The etiology of situs inversus is uncertain, but recent evidence suggests that genetic alterations leading to abnormal cilia may be the causative factor [5]. Patients with situs inversus may present with confusing clinical symptoms, for example, left lower quadrant pain in the setting of acute appendicitis. Situs abnormalities overall are a complex topic and may be associated with other abnormalities such as congenital heart disease [6].
Computed Tomography Techniques for Imaging the Colon
Routine CT of the abdomen and pelvis with intravenous and oral contrast material is adequate for evaluating the colon for most indications [7] as it provides excellent visualization of the colonic wall as well as of adjacent structures.
Post-contrast images are typically acquired in the portal venous phase of contrast enhancement which occurs approximately 60–70 s after intravenous contrast material administration. Specific protocols tailored to look for sites of gastrointestinal bleeding also have been developed and typically utilize noncontrast, arterial phase, and delayed phase imaging [8, 9].
Oral contrast agents are categorized as “positive” (appearing hyperattenuating on CT scans) or “negative” (appearing hypoattenuating on CT scans). Options for positive oral contrast agents include water-soluble iodine-based agents (e.g., Gastrografin (Bracco Diagnostics, Princeton, NJ)) and barium-based agents. With Gastrografin, patients typically consume approximately 900–1,000 mL of a mixture of 2.5 % diluted sodium amidotrizoate and meglumine amidotrizoate with images acquired approximately 30–60 min after ingestion of contrast material [7, 10]. Similar volumes and imaging times also are used for barium-based agents.
The primary advantage of a water-soluble agent is its safety in patients with bowel perforations. Specifically, an extravasated water-soluble contrast material will be resorbed by the body whereas extravasated barium is not (Fig. 12.5). Patients should be screened for iodine allergy prior to the administration of water-soluble contrast agents that contain iodine.
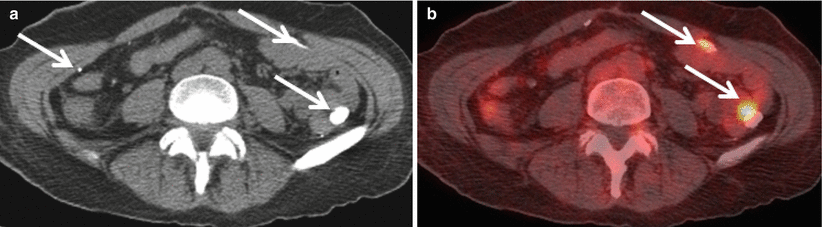

Fig. 12.5
Axial contrast enhanced CT (a), demonstrates extravasated barium (arrows) in a patient with a large rectal perforation who was administered with a barium-based oral contrast material at an outside institution several months prior to this PET/CT. Axial fused FDG PET demonstrates inflammation associated with extravasated barium was hypermetabolic at PET imaging (b, arrows) and difficult to distinguish from metastatic disease
Recent literature suggests that oral contrast material may not be necessary in certain settings such as oncology patients undergoing follow-up examinations [11] and trauma patients [12]. The advantages of eliminating oral contrast material from imaging protocols include eliminating the 30–60 min delay between contrast material ingestion and imaging. Also, some patients find the taste of oral contrast material objectionable, and allergic reactions to oral contrast agents have been reported. However, an oral contrast material is required to demonstrate colonic leaks and fistulas (Fig. 12.6).
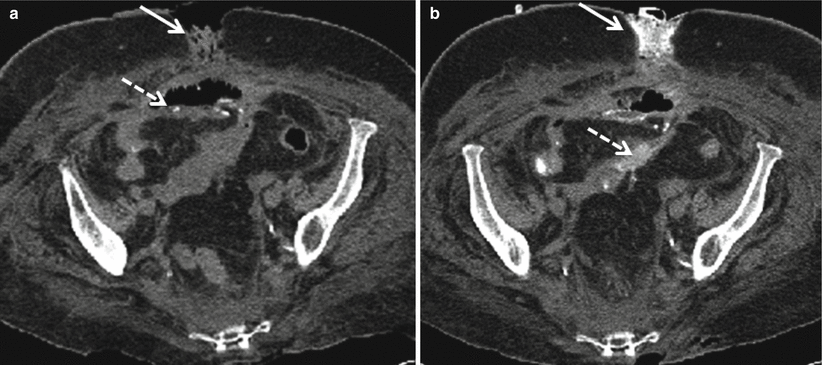

Fig. 12.6
Importance of oral contrast material in demonstrating bowel leaks and fistula. Initial noncontrast CT demonstrates open ventral wound (a, solid arrow) and ileocolic anastomosis (a, dashed arrow). Repeat imaging obtained after administration of oral contrast material demonstrates extravasated contrast material exiting the open wound in keeping with a coloatmospheric fistula (b, solid arrow) as well as oral contrast material leaking into the peritoneal cavity (b, dashed arrow)
Negative oral contrast agents (e.g., VoLumen (Bracco Diagnostics, Princeton, NJ) allow for improved visualization of the bowel wall with CT enterography protocols [13]. CT colonography (see Chap. 11) is the preferred method for evaluating colonic mucosal abnormalities such as polyps.
Rectally administered contrast material may help in the evaluation of colonic pathology by producing increased colonic distention and opacification as compared to orally administered contrast material. A rectal contrast material may be administered using a rectal catheter with 400–1,000 mL of a 4 % iothalamate meglumine solution (Conray; Mallinckrodt, St. Louis, MO) instilled under force of gravity [14].
Algorithmic Approach
The three major categories of colonic pathology detectable at cross-sectional imaging include colonic wall thickening, colonic masses, and colonic dilatation. An algorithmic approach is presented for each below (Algorithm 12.1).
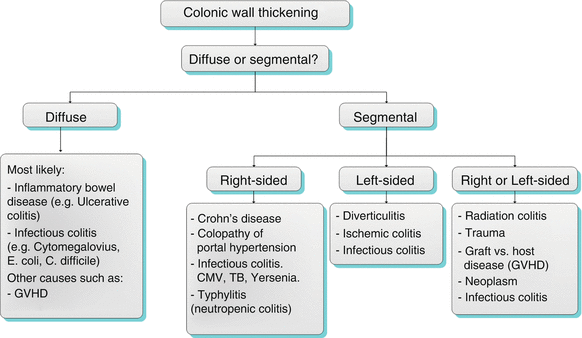

Algorithm 12.1
Colonic wall thickening can be either diffuse, focal or segmental. Focal thickening is predominantly noted secondary to mass (discussed below). The most common etiologies of diffuse thickening include inflammatory bowel disease (ulcerative colitis which is a characterized by contiguous disease, as opposed to Crohn’s disease which is characterized by skip lesions. Infectious colitis (such as C. defficile, CMV and E. Coli) Location is the most important diagnostic clue for segmental wall thickening (which is non-specific in the majority of cases). The right colon is typically involved in Crohn’s, portal hypertension, infectious colitis and typhilitis. The left side is typically involved in diverticulitis, & ischemic colitis (splenic flexure or sigmoid). Either side can be involved in various other conditions
Colonic Wall Thickening
Colonic wall thickening may be due to a variety of etiologies including infection, inflammation, and neoplasm. Ultimately, correlation with laboratory tests including stool studies, as well as clinical history, and histologic analysis may be required to confirm the diagnosis.
However, evaluating whether colonic wall thickening is diffuse or segmental and, if segmental, the location of wall thickening helps narrow the differential diagnosis [15]. For example, diffuse colonic wall thickening extending from the rectum may be due to ulcerative colitis, whereas wall thickening isolated to the right colon in a cirrhotic patient may reflect colopathy of portal hypertension. An algorithmic approach is presented below with each entity discussed in the following paragraphs:
Inflammatory Bowel Disease
Inflammatory bowel diseases are characterized by a relapsing course. The most common inflammatory bowel diseases are ulcerative colitis and Crohn’s disease.
Ulcerative Colitis
The etiology of ulcerative colitis (UC) is uncertain but is thought to be a combination of genetic and environmental factors [16]. Individuals with UC typically present with persistent diarrhea, sometimes with fever, pain, and weight loss, and are usually between the ages of 15 and 40 when diagnosed [16].
Ulcerative colitis is a superficial inflammatory process with near-obligatory rectal involvement. Ulcerative colitis typically progresses distally to proximally in a continuous fashion unlike the skip lesions often seen with Crohn’s disease. Primary small bowel involvement with ulcerative colitis is rare, and there can rarely be secondary involvement of the terminal ileum in the setting of pancolitis (so called backwash ileitis).
Active disease due to ulcerative colitis is characterized by hyperemia of the bowel wall, mural stratification, and adjacent inflammatory change (Fig. 12.7) [7]. Mural stratification occurs because bowel wall edema results in alternating layers of enhancing muscosa and serosa with intervening edematous hypoattenuating submucosa after the administration of intravenous contrast material.
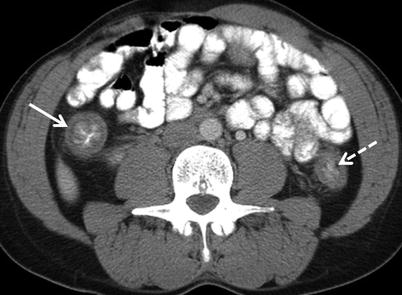

Fig. 12.7
Ulcerative colitis – active disease. Active disease manifests as contiguous wall thickening extending from the rectum, involving also the ascending (white arrow) and descending colon (white dashed arrow) in this patient
Unlike Crohn’s disease, fistula formation is rare with ulcerative colitis as ulcerative colitis is more of a superficial mucosal process. In individuals with severe, fulminant ulcerative colitis, extensive coalescent mucosal sloughing may occur [16]. Remaining islands of relatively normal mucosa appear as pseudopolyps surrounded by areas of sloughed mucosa [16].
Over time, hypertrophy of the muscularis mucosa results in a narrowed and ahaustral colon pattern, also known as “lead pipe colon,” and submucosal fat deposition may occur (Fig. 12.8). Submucosal fat deposition (“fat halo sign”) may also occur in patients with other forms of colitis and in the obese [17].
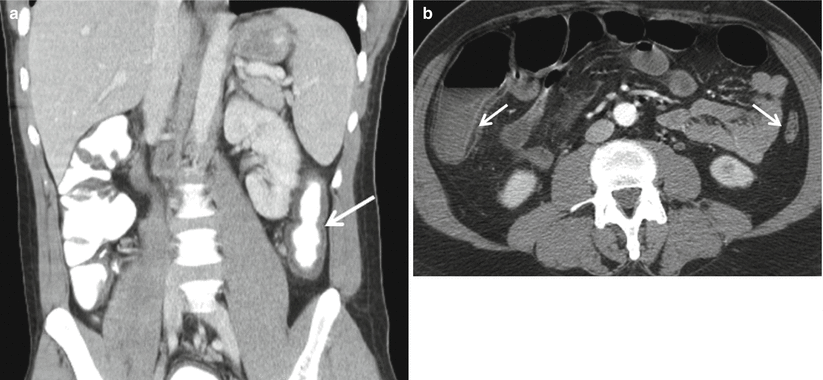

Fig. 12.8
Sequelae of ulcerative colitis. Over time, individuals with ulcerative colitis may develop an ahaustral pattern of involved segments of the colon (“lead pipe colon”) as seen in the left colon (a, arrow) in this coronal image. Submucosal fat deposition may also occur (b, arrows)
Complications associated with ulcerative colitis include toxic megacolon and development of colonic adenocarcinoma. Toxic megacolon can be a fatal complication of acute flairs of ulcerative colitis but, fortunately, is seen in fewer than 5 % of ulcerative colitis patients [16]. Toxic megacolon is defined as dilatation of a nonobstructed colon to >6 cm in diameter with or without signs of systemic toxicity [18]. At CT, toxic megacolon appears as a dilated colon which may be thin walled (Fig. 12.9) [7]. Toxic megacolon also can be seen with other types of colitis including infectious colitis.
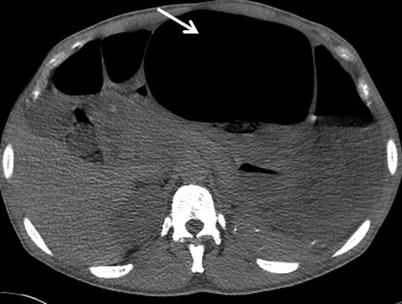

Fig. 12.9
Toxic megacolon. Axial non-enhanced CT demonstrates marked dilatation of the transverse colon to 10 cm in diameter (arrow)
Patients with long-standing ulcerative colitis also are at risk for colonic adenocarcinomas with an 18 % incidence of adenocarcinoma by age 30 in one meta-analysis [19]. For this reason, patients with ulcerative colitis often undergo prophylactic sphincter-sparing total colectomy. Approximately 5 % of patients with ulcerative colitis will develop primary sclerosing cholangitis [20].
Key Imaging Features of Ulcerative Colitis
1.
Rectal involvement with proximal extension in a continuous fashion.
2.
Active disease: Mural stratification and adjacent stranding.
3.
Severe, fulminant cases demonstrate pseudopolyps.
4.
Toxic megacolon (>6 cm in diameter without obstruction) is a fatal complication occuring in less than 5 %.
5.
Chronic changes include ahaustral (“lead pipe”) colon and submucosal fat deposition.
6.
Increased risk for colonic adenocarcinoma and primary sclerosing cholangitis.
Crohn’s Disease
The etiology of Crohn’s disease is uncertain but is thought to result from a combination of genetic and environmental factors [21]. Patients with Crohn’s disease generally present with persistent diarrhea and abdominal pain and are most often diagnosed between the ages of 15 and 30 [21].
Crohn’s disease most commonly involves the terminal ileum and proximal colon [22]. Isolated colonic involvement occurs in 15–20 % of patients, and colonic involvement occurs along with small bowel involvement in 50 % of cases [23].
Crohn’s disease ranges in severity from relatively mild cases of mucosal inflammation to severe cases of transmural inflammation with fistula formation [22]. Early mucosal abnormalities are best depicted at colonoscopy and with barium studies.
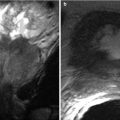
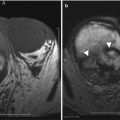
CT and MR are valuable for evaluating active disease in patients with transmural involvement. At CT, active Crohn’s disease is characterized by wall thickening, hyperemia, mural stratification, and surrounding inflammatory changes [22] (Fig. 12.10). The “comb sign” indicating prominent vascular structures may be present [24] (Fig. 12.10). MR can also demonstrate wall edema (submucosal low signal intensity on T1-w and high signal intensity on T2-weighted images) and hyperemia resulting in mucosal hyperenhancement (Fig. 12.11).
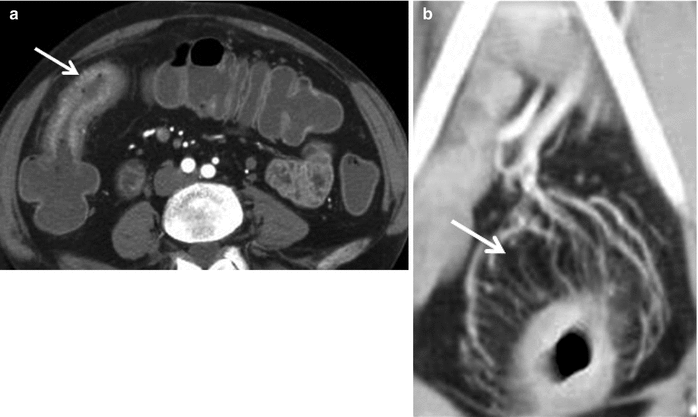
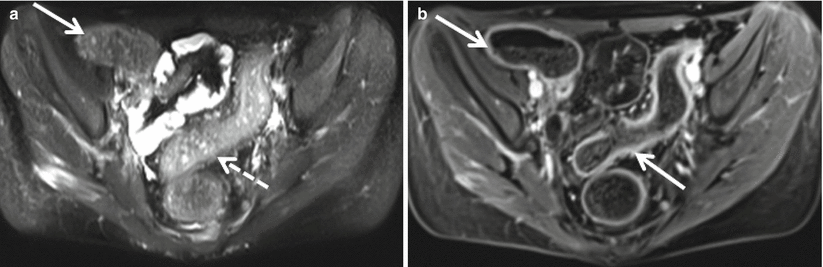

Fig. 12.10
Active Crohn’s disease. Axial contrast enhanced CT enterography (a) demonstrates wall thickening (arrow), mucosal hyperenhancement and mural stratification. Coronal reformatted image of contrast enhanced CT (b) demonstrates active Crohn’s disease involving the rectum, with dilated vascular arcades “Comb sign” (arrow)

Fig. 12.11
Active Crohn’s disease. Axial contrast enhanced CT enterography (a) demonstrates wall thickening (arrow), mucosal hyperenhancement and mural stratification. Coronal reformatted image of contrast enhanced CT (b) demonstrates active Crohn’s disease involving the rectum, with dilated vascular arcades “Comb sign” (arrow)
CT and MR are also helpful in evaluating complications such as the development of fistulae (Fig. 12.12), abscesses, and fibrostenotic strictures (Fig. 12.13) [23]. Attention should be paid to the entire bowel when interpreting cross-sectional imaging studies of patients with Crohn’s disease as “skip lesions” (discontinuous segments of diseased bowel with intervening normal segments) are characteristic of the disease process.
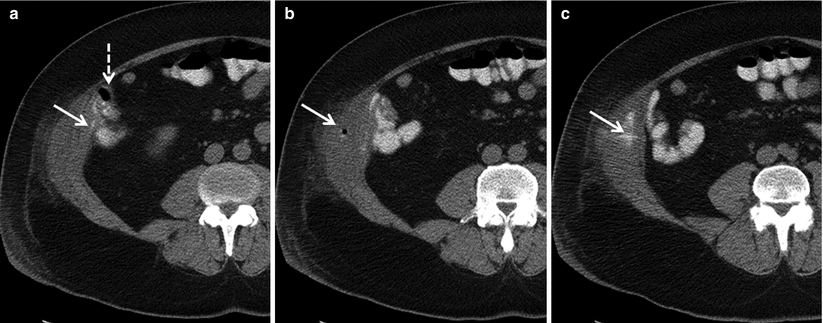
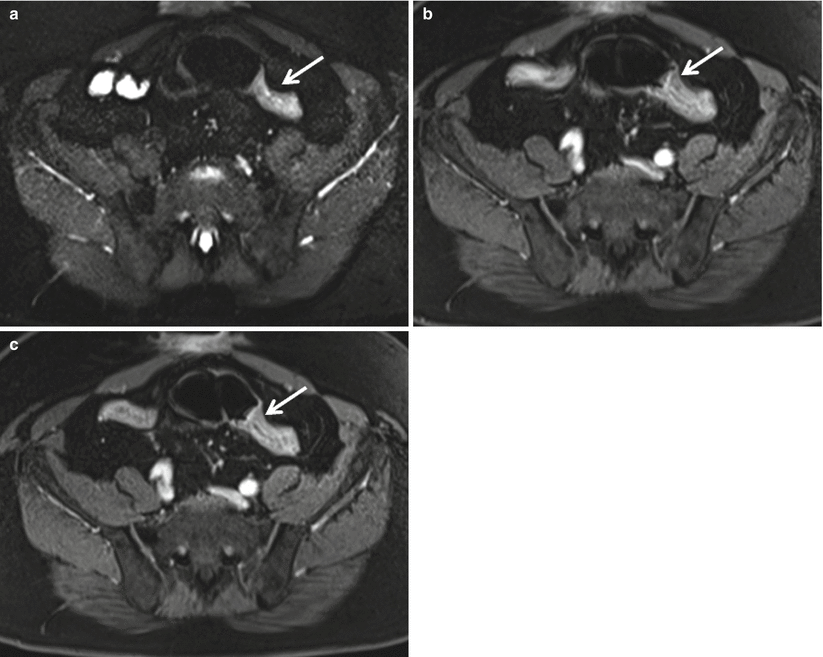

Fig. 12.12
Colonic fistula due to Crohn disease. Axial contrast enhanced CT demonstrates extravasated oral contrast material (solid arrow) tracking away from the cecum (dashed arrow) (a), with collection of air (b, arrow) and contrast material (c, arrow) in the abdominal wall musculature

Fig. 12.13
Chronic fibrotic stricture due to Crohn’s disease. Note segmental wall thickening of the sigmoid colon (arrows). T2 image with fat saturation (a) demonstrates no surrounding acute inflammatory changes. Post-contrast venous phase (b) and 3-min delay images (c) demonstrate delayed hyperenhancement reflecting fibrous tissue
Special attention also should be paid to the perianal area in patients with known or suspected Crohn’s disease as the presence of perianal abscesses (Fig. 12.14) is supportive of a diagnosis of Crohn’s disease in the appropriate clinical setting. MR is the modality of choice for characterizing perianal fistulae given the increased soft tissue resolution possible with MR as compared to CT (Fig. 12.15) [25].
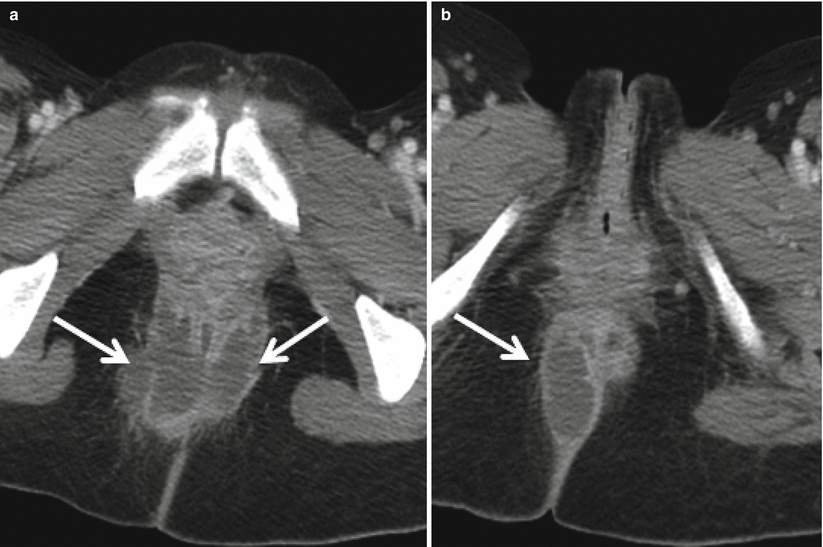
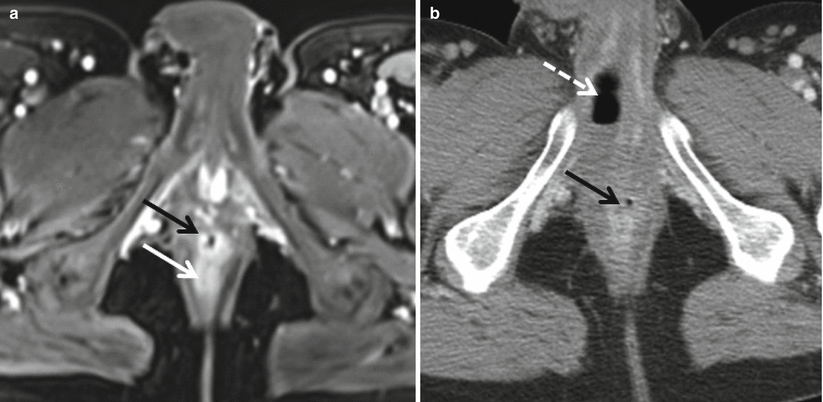

Fig. 12.14
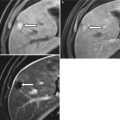
Axial contrast enhanced CT at the level of anal canal (a, b) demonstrates well circumscribed rim enhancing perianal fluid collections (arrows), most compatible with perianal abscesses in this patient with Crohn’s disease

Fig. 12.15
Perianal abscess. Axial contrast enhanced T1-weighted image demonstrates a rim-enhancing abscess (black arrow) originating along the anterior wall of the anus (white arrow). Axial contrast-enhanced CT shows inflammatory changes (solid arrow), with an air containing abscess (dashed arrow) within the base of the penis
Chronic fibrotic changes may also produce wall thickening at CT. Lack of adjacent inflammatory change and a more homogeneous appearance of the bowel wall as opposed to the mural stratification seen with acute inflammation can help distinguish wall thickening due to chronic fibrosis from wall thickening due to acute inflammation. Delayed hyperenhancement of an area of wall thickening seen at dynamic contrast-enhanced MR enterography also indicates chronic fibrotic change (Fig. 12.13) [26].
Cross-sectional imaging plays a key role in determining which patients may benefit from surgical intervention. Indications for surgery include perforation, obstructing fibrostenotic strictures, and fistulae that do not respond to medical management [23].
Key Imaging Features of Crohn’s disease
1.
Terminal ileum is typically involved.
2.
Skip lesions.
3.
Colonic inflammation may occur with or without small bowel inflammation.
4.
Can by complicated by perianal abscesses, fistula formation and strictures.
5.
Bowel wall thickening due to active disease is characterized by mural stratification, mucosal hyperenhancement, and surrounding inflammatory change.
6.
Bowel wall thickening due to chronic fibrosis characterized by homogeneous attenuation of the wall and delayed hyperenhancement at CT or MR enterography.
Infectious Colitis
A wide variety of infections including bacterial (e.g., M. tuberculosis, Shigella, Yersinia, Staphylococcus, Salmonella, Campylobacter), fungal (e.g., actinomycosis and histoplasmosis), and viral (e.g., Rotavirus, Cytomegalovirus, and herpes) can result in colitis [27]. Laboratory tests including stool studies are required to determine a specific organism as imaging features are nonspecific.
At CT, infectious colitis appears as colonic wall thickening, often with surrounding inflammatory change that may be indistinguishable from other causes of colitis [27]. Therefore, clinical correlation is key. Although not definitive, distribution of wall thickening may provide additional information regarding the possible causative organism. For example, cytomegalovirus and E. coli more commonly result in a pancolitis (Fig. 12.16), whereas M. tuberculosis, Shigella, and Salmonella may be limited to the right colon [27, 28].
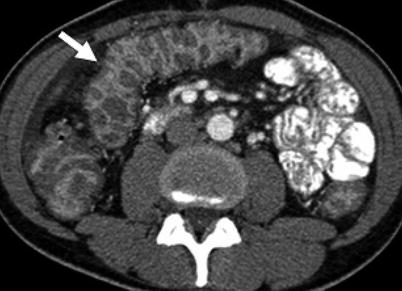

Fig. 12.16
Axial contrast enhanced CT demonstrates marked colonic wall thickening (arrow) in an HIV-positive patient who presented with abdominal pain. Colonoscopic biopsy demonstrated cytomegalovirus colitis
Pseudomembranous colitis gets its name from the characteristic yellowish plaques which form along the colonic mucosa and is caused almost exclusively by toxins produced by C. difficile [29]. This infectious colitis most commonly affects elderly, hospitalized patients as a complication of antibiotic therapy. At CT, patients may exhibit diffuse or segmental colonic wall thickening (Fig. 12.17) [30, 31]. Some series [30] report diffuse wall thickening as being more common while other series [31] report that segmental wall thickening is more common. When segmental wall thickening is observed with pseudomembranous colitis, any segment of the colon may be involved, with rectosigmoid involvement reported as being the most common in one series [31]. As with ulcerative colitis, patients with pseudomembranous colitis may also develop toxic megacolon, and severe C. difficile infection may be fatal.
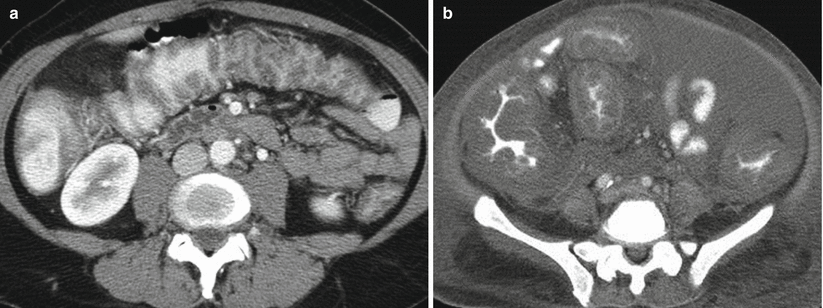

Fig. 12.17
Infectious colitis. Axial contrast enhanced CT in two patients (a and b) with pancolitis and stool studies positive for C. difficile toxin in keeping with pseudomembranous colitis due to C. difficile infection. Note marked colonic wall thickening involving the entire colon
Key Imaging Features of Infectious Colitis
1.
Colonic wall thickening, often with adjacent inflammatory changes.
2.
May be indistinguishable from other causes of colitis based on imaging alone.
3.
Laboratory tests including stool studies are necessary to determine the causative organism.
4.
Pseudomembranous colitis may be a pancolitis or segmental.
Colopathy of Portal Hypertension
Colopathy of portal hypertension should be considered in patients with wall thickening of the cecum and ascending colon. Right-sided colonic wall thickening may be seen in >30 % of patients with cirrhosis [32]. The etiology is thought to be altered hemodynamics due to portal hypertension [32]. Of cirrhotic patients with right-sided colonic wall thickening, >90 % also had varices and ascites and nearly 90 % had splenomegaly in one series [32].
At CT, right colon wall thickening without adjacent inflammatory changes is most likely due to colopathy of portal hypertension if the patient has evidence of chronic liver disease and other findings of portal hypertension such as ascites, splenomegaly, and varices (Fig. 12.18).
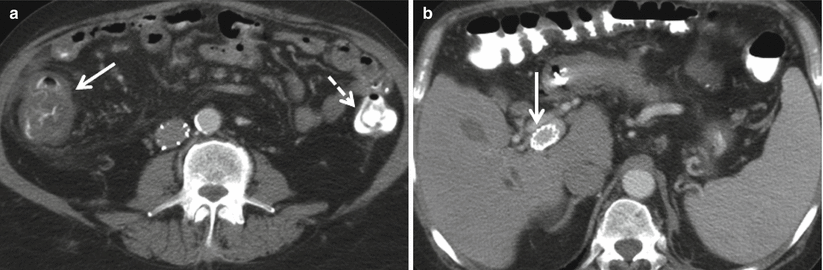

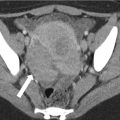
Fig. 12.18
(a, b)Colopathy of portal hypertension. Axial contrast enhanced CT demonstrate wall thickening of the right colon (a, solid arrow) and normal appearance of the left colon (a, dashed arrow) in this patient with cirrhosis, portal hypertension, and a transjugular intrahepatic portosystemic shunt (TIPS, b arrow)
Colopathy of portal hypertension is asymptomatic and usually improves in patients who undergo liver transplant [32]. In cirrhotic patients with asymptomatic right colon wall thickening on CT, colonoscopy may demonstrate a normal mucosa or mucosa with increased vascularity and telangiectasia [33]; the latter is termed “portal hypertensive intestinal vasculopathy” in the gastroenterology literature and requires no further follow-up [34].
Key Imaging Features of colopathy secondary to portal hypertension
1.
Right colon wall thickening in a patient with cirrhosis and evidence of portal hypertension.
2.
Vast majority of patients will also demonstrate ascites, varices, and/or splenomegaly.
Typhlitis
Typhlitis should be considered in patients with cecal wall thickening. Typhlitis (also known as neutropenic colitis) is a necrotizing right-sided colitis involving the cecum and sometimes the adjacent ascending colon and terminal ileum [27, 35]. The etiology of typhlitis is uncertain, but bacterial invasion of the bowel wall may be a cause with secondary necrosis, bacteremia, and possible bowel perforation [35]. The reason for cecal predominance of this disease process is unclear.
This potentially fatal condition occurs in neutropenic patients whose bone marrow is suppressed due to illness or treatment [35]. Typhlitis has been reported in patients with myelodysplastic syndrome, aplastic anemia, multiple myeloma, and AIDS, as well as in patients who have undergone solid organ or bone marrow transplantation [35]. Patients present with abdominal pain and fever [35], and laboratory values are usually nonspecific.
At CT, typhlitis appears as a focal cecal wall thickening with surrounding inflammatory changes (Fig. 12.19) [27]. Complications visible at CT include pneumatosis and adjacent abscesses [27].
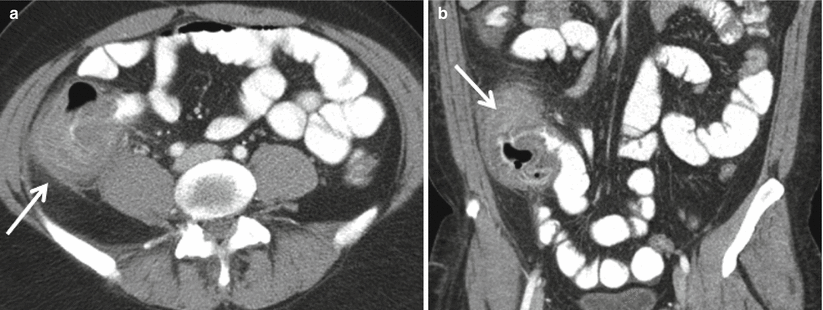

Fig. 12.19
Typhlitis. Axial (a), and coronal reformatted (b) images of contrast enhanced CT demonstrate focal cecal wall thickening (arrows) in this bone marrow transplant patient with fever and right lower quadrant pain
Medical management including antibiotics and supportive care is the first-line treatment with surgery required in some severe cases [35].
Key Imaging Features of typhlitis
1.
Cecal wall thickening in a neutropenic patient.
2.
The ascending colon and terminal ileum also may be involved.
Diverticulitis
Though diverticulitis can occur anywhere along the colon, it is most commonly a left-sided process. Colonic diverticula are outpouchings of colonic mucosa and submucosa through the muscular wall of the colon [27]. Diverticula can occur anywhere along the colon (Fig. 12.20) but are usually greatest in number along the sigmoid colon. “Diverticulosis” refers to the presence of uninflamed diverticula. “Diverticulitis” refers to the presence of inflamed diverticula.
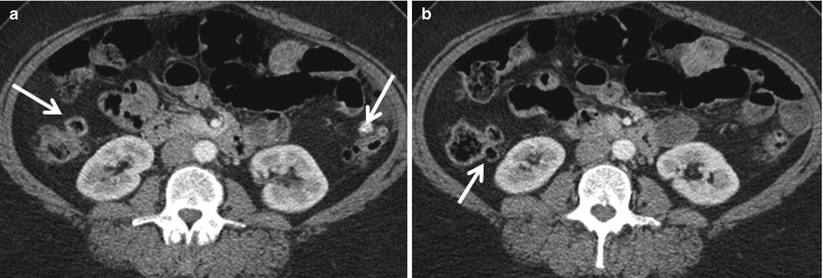

Fig. 12.20
Pandiverticulosis. Axial contrast enhanced CT (a, b) demonstrates numerous diverticular outpouchings along the entire colon including the ascending and descending colon (arrows)
The term “complicated diverticulitis” is, in general, used when abscess formation, gross perforation, peritonitis, or bowel obstruction accompanies the episode of acute diverticulitis [36].
The exact pathophysiology of diverticulitis is uncertain but is thought to be due to stasis, bacterial overgrowth, and narrowing or obstruction of the neck of the diverticulum [36]. Clinically, patients with acute diverticulitis present with abdominal pain which is often left sided. Patients also may experience nausea, vomiting, anorexia, low-grade fever, and leukocytosis [36].
At CT, acute diverticulitis appears as a segmental colonic wall thickening with inflammation of the adjacent fat [27] (Fig. 12.21). Identification of diverticula in the area of colonic wall thickening helps distinguish diverticulitis from other causes of colonic wall thickening [27]. CT imaging is highly sensitive (>95 %) and specific (>95 %) for the diagnosis of acute diverticulitis [36]. Additionally, CT can diagnose complications of diverticulitis such as abscess formation (Fig. 12.22), bowel obstruction, and fistula formation (Fig. 12.23). The presence or absence of these complications alters patient management.
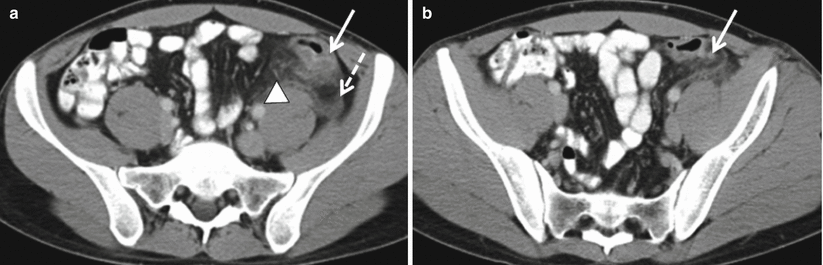
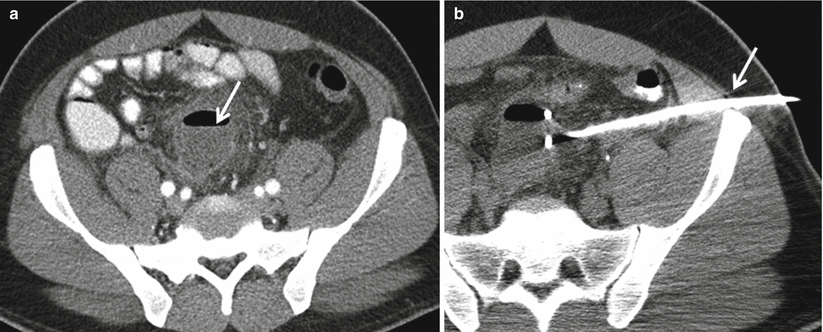
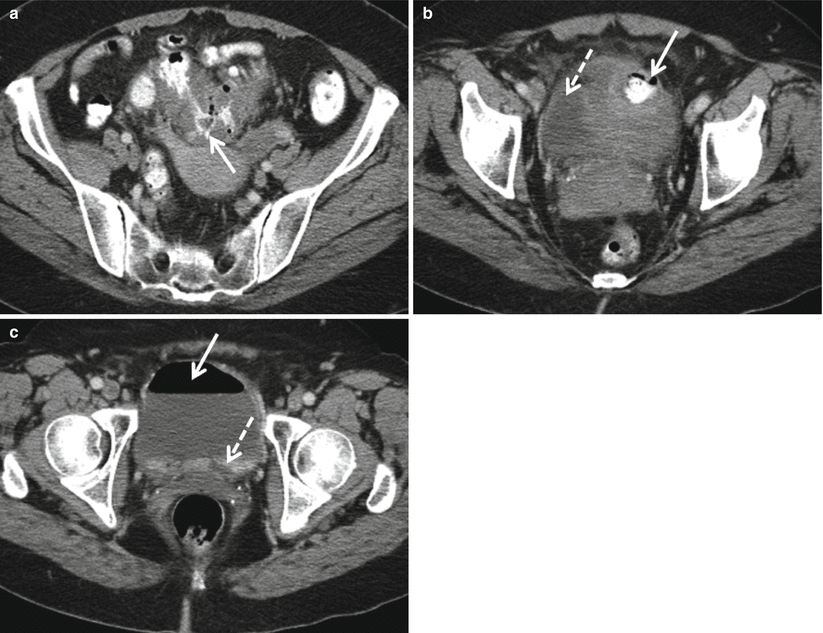

Fig. 12.21
Uncomplicated acute diverticulitis. Axial contrast enhanced CT demonstrates segmental wall thickening involving the sigmoid colon (a, solid arrow) with adjacent fluid (a, dashed arrow) and vascular engorgement (a, arrow head) in an area of diverticular disease (b, arrow)

Fig. 12.22
Complicated diverticulitis, percutaneous drainage. Axial contrast enhanced CT demonstrates and air-containing abscess (a, arrow) related to acute diverticulitis (not shown). CT-guided percutaneous drainage catheter placement into abscess (b, arrow)

Fig. 12.23
Complicated diverticulitis ultimately requiring surgery. Axial contrast enhanced CT demonstrates thick-walled, inflamed segment of the sigmoid colon (a, arrow) in an area of diverticular disease. Extravasation of oral contrast material into a thick-walled abscess (b, arrow) along the bladder (b, dashed arrow) dome. Note air (c, arrow) and oral contrast material (c, dashed arrow) confirming a colovesicular fistula that ultimately required surgical repair following an extended course of antibiotics and CT-guided percutaneous drainage of the abscess
Management ranges from outpatient management with antibiotics for patients with uncomplicated diverticulitis to hospitalization and surgery for patients with complicated diverticulitis. Imaging plays a key role in determining management. For example, patients who are able to tolerate oral intake can be managed as outpatients with antibiotics [36]. Patients with small (<4 cm) abscesses without peritonitis may be managed with antibiotics and bowel rest, whereas patients with larger abscesses may benefit from image-guided percutaneous drainage (Fig. 12.22) [36]. Indications for surgery include lack of response to medical management, fistula formation, obstruction, free perforation, or repeated attacks [36].
Key Imaging Features of acute diverticulitis
1.
Focal or segmental wall thickening with pericolonic fat stranding involving a segment of the colon with diverticula.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree