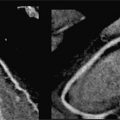
FIGURE 6-1 Precordial leads from an eCG performed as part of a routine checkup in a 43-year-old man demonstrate marked T-wave abnormality, particularly in V3 through V6.
Family history revealed hypertension in his father aged 68 years as well as in the 7 siblings of his father. One paternal uncle died suddenly at 32 due to a ruptured cerebral aneurysm. Patient disclosed prior cardiac evaluation to include a TTE that was reported as normal, as well as a negative stress ECHO. Based on the ECG abnormalities, CMR examination was performed.
Breath-held SSFP cine imaging was obtained and demonstrated apical hypertrophy (Figures 6-2 and 6-3, Video 6-1). There was no flow acceleration across the LV outflow track and no systolic anterior motion of the mitral valve. Late gadolinium enhancement (LGE) imaging (Figure 6-4) showed patchy myocardial hyper-enhancement of the hypertrophied apical myocardium. Some hyperenhancement was also seen in the septum and lateral walls.
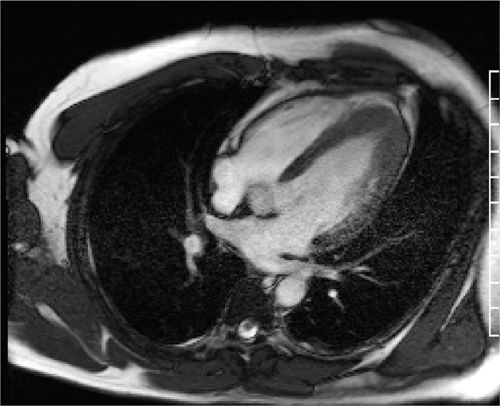
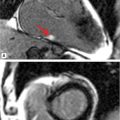
FIGURE 6-2 Diastolic frame from a horizontal long-axis cine acquisition shows prominent thickening of the apical-LV myocardium.
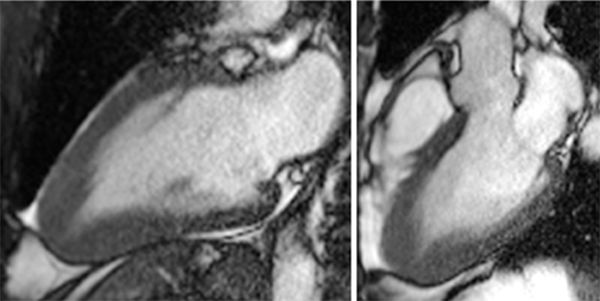
FIGURE 6-3 End-diastolic frames from vertical long-axis (left) and 3CH (right) cine acquisitions show marked thickening of the apical LV myocardium.
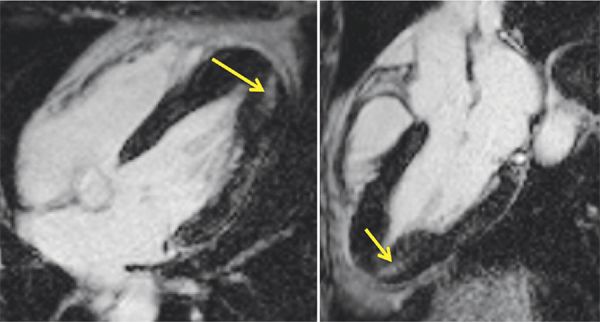
FIGURE 6-4 Late gadolinium enhancement images obtained using an inversion recovery-prepared gradient-echo sequence 10 to 15 minutes after intravenous administration of contrast demonstrates patchy hyperenhancement most evident in the inferior apical myocardium (arrows) in horizontal long-axis (left) and 3-CH (right) planes. Some hyperenhancement was also evident along the lateral wall.
Discussion
Cardiac magnetic resonance is a powerful tool for evaluation of patients with known or suspected hypertrophic cardiomyopathy (HCM). A typical protocol for HCM includes cine imaging, LGE acquisitions, and VENC to assess for outflow tract obstruction, and severity of concomitant valvular disease. Presence and extent of hypertrophy as well as patterns of myocardial enhancement by LGE imaging may help establish the diagnosis.
The apical variant of HCM may be suspected based on ECG abnormalities of deep T-wave inversion and increased QRS voltage across the precordial leads. Apical HCM may be missed with TTE due to inadequate visualization of the apex of the heart.1 Cardiac magnetic resonance warrants consideration in the HCM patient for risk stratification for sudden cardiac death or heart failure. Prospective studies support that the risk of heart failure admissions, heart failure related death or symptomatic heart failure (New York Heart Association [NYHA] Class III or IV) is statistically increased in HCM patients with fibrosis and as the fibrosis increases the risk increases.2
CASE 2 Septal Hypertrophic Cardiomyopathy
A 44-year-old man with a past medical history of atrial fibrillation and hyperlipidemia presented to an emergency department with complaints of chest pain. He described the pain as substernal with radiation to left arm and jaw with associated diaphoresis. Based on his symptoms as well as a markedly abnormal ECG with deep T-wave inversions in the septal and lateral leads, he underwent cardiac catheterization. He had no obstructive CAD, but hyderdynamic LV systolic function was noted. Additional history indicated a single episode of syncope of unclear etiology. Family history included dilated cardiomyopathy in his father who died at the age of 69 and cardiomyopathy in 2 living paternal uncles.
A TTE was performed and was consistent with a diagnosis of HCM with maximal septal wall thickness of 2.4 cm. Based on his high-risk features of prior syncope, he warranted ICD placement. Prior to that, a cardiac MRI was obtained for assessment of myocardial morphology and scar.
Cine imaging (Figure 6-5) showed marked septal hypertrophy, maximal end-diastolic thickness of 2.9 cm. Late gadolinium enhancement imaging (Figure 6-6) showed typical foci of mid-myocardial enhancement in the hypertrophied septum. In-plane VENC imaging performed in the 3-CH view showed no evidence of systolic anterior motion of the mitral valve and no outflow gradient at rest despite asymmetric septal hypertrophy (Video 6-2). This is in comparison to an HCM patient with dynamic left ventricular outflow tract (LVOT) obstruction and systolic anterior motion of the mitral valve (Video 6-3).
FIGURE 6-5 A still frame from an SSFP breath-held cine acquisition in the horizontal long-axis plane shows marked septal hypertrophy, maximal thickness of 2.9 cm.
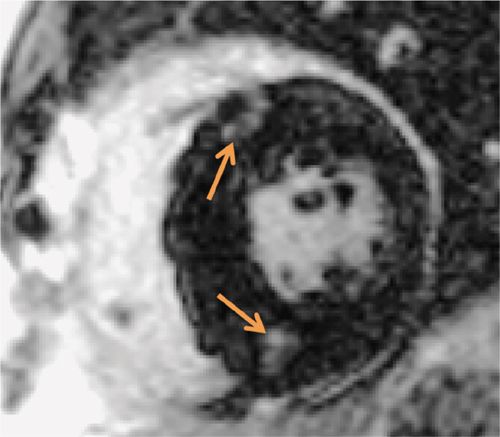
FIGURE 6-6 Late post-gadolinium imaging acquired in a short-axis plane demonstrates the typical foci of mid-myocardial enhancement (arrows) in the hypertrophied interventricular septum.
Discussion
Cardiac magnetic resonance is useful for characterization of HCM due to better image quality and spatial resolution myocardial fibrosis assessment. It is helpful for identification of the level of obstruction, especially with patients with suboptimal transthoracic acoustic windows. The technique can rule out the presence of a subaortic membrane, another cause of LVOT gradient, and can assist in further characterization of the mitral valve anatomy and systolic anterior motion of the mitral valve.3 If clinical features such as syncope, family history of sudden death in a relative under the age of 50, and ventricular arrhythmia are absent, CMR may be helpful to define severity of hypertrophy and extent of fibrosis to guide decision-making regarding ICD placement.4
CASE 3 Troponin-T Type-2 Gene (TNNT2) Mutation
A 49-year-old woman with palpitations and ECG monitoring that showed paroxysms of atrial fibrillation presented for cardiovascular evaluation. She disclosed a family history of HCM with at least 6 family members with sudden cardiac death. This included her mother, 2 of her mother’s siblings, and several maternal cousins. The patient’s daughter had been diagnosed with restrictive cardiomyopathy and was under consideration for cardiac transplantation. Echocardiography showed borderline hypertrophy and atrial enlargement. Based on the significant family history she underwent genetic testing. She was found to be positive for a mutation in the TNNT2 gene. Given her genetic abnormality of a mutation with a high risk of sudden death plus numerous relatives with sudden cardiac death, there was a high index of suspicion that she had HCM despite borderline TTE findings. Therefore, CMR was obtained to further evaluate for cardiomyopathy.
Cine imaging showed mild septal thickening, measuring up to 1.3 cm, as well as focal deformity and crypt in the inferior LV myocardium (Figure 6-7, Video 6-4). Late post-gadolinium imaging showed no significant hyperenhancement (Figure 6-8). Collectively, these morphological abnormalities were consistent with a diagnosis of HCM without obstruction.
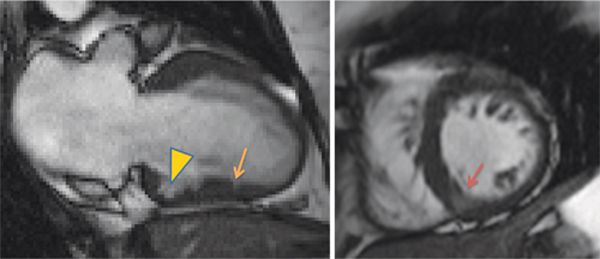
FIGURE 6-7 Frame from a vertical long-axis SSFP cine acquisition (left) in a patient with TNNT2 mutation, atrial fibrillation, and a family history of sudden cardiac death shows a small crypt in the apical inferior wall. A focal deformity of the basal inferior wall is also seen (arrowhead). Basal short-axis cine frame (right) shows mild thickening of the septum, maximum thickness 1.3 cm. Note that partial volume effect makes the inferior septum appear relatively bright (dark orange arrow) due to presence of blood and myocardium over the 8 mm slice.
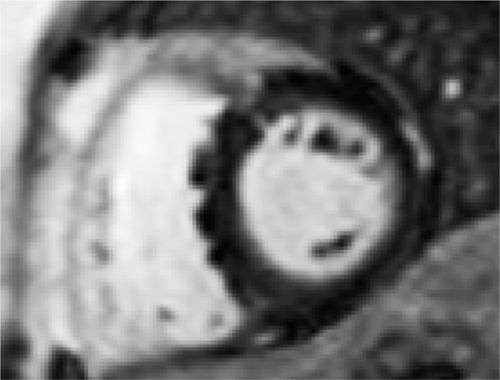
FIGURE 6-8 Late post-gadolinium imaging acquired in a short-axis plane shows no significant myocardial hyperenhancement. Partial volume averaging of blood amidst RV endocardial trabeculations along the inferior septum should not be mistaken for mid-myocardial enhancement of the septum.
Discussion
The TNNT2 gene encodes for a protein for the tropomyosin-binding subunit of the troponin complex. Located on the thin filament, it responds to changes in intracellular calcium concentration. Mutations in this gene are associated with a variety of phenotypic expressions including hypertrophic, dilated and restrictive cardiomyopathies. Cardiac magnetic resonance is a useful adjunct to genetic testing with its unique ability to define subtle phenotypic changes of HCM such as myocardial crypts.5, 6
CASE 4 Anderson-Fabry Disease
A 62-year-old woman presented with generalized edema and symptoms of peripheral neuropathy. Laboratory testing including 24-hour urine analysis indicated nephrotic syndrome, prompting renal biopsy that was diagnostic of Anderson-Fabry Disease (AFD). Due to the associated cardiac involvement with Fabry disease, CMR examination was performed.
Cine imaging demonstrated mild concentric remodeling (Figure 6-9) and normal LV systolic function; calculated EF was 73%. Late post-gadolinium imaging demonstrated a focus of hyperenhancement involving the lateral apex (Figure 6-9). This was felt to represent cardiac involvement of AFD; follow-up CMR examination 2 years after initiation of enzyme replacement therapy has shown no progression of LGE-positive extent or LV thickening.
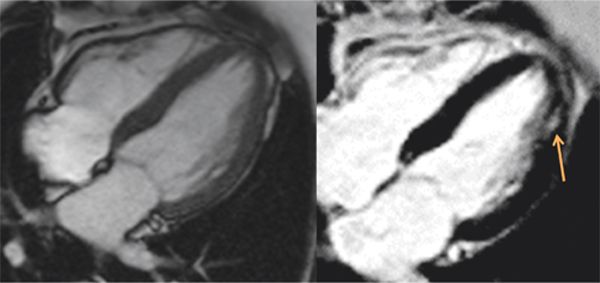
FIGURE 6-9 Cine frame (left) shows mild LV thickening in a patient with Anderson-Fabry disease and normal blood pressure. Late gadolinium imaging in the same plane (right) shows a focus of mid-myocardial enhancement of the lateral apex (arrow).
Discussion
Cardiac involvement with metabolic storage diseases is common. Typical cardiac features include left ventricular hypertrophy (LVH) and restrictive filling patterns, with clinical sequelae including congestive heart failure (CHF) and ventricular arrhythmias (VAs). The cardiomyopathy associated with AFD typically progresses from concentric remodeling to concentric hypertrophy. Some patients may demonstrate asymmetric septal hypertrophy (5% of cases). Cardiac magnetic resonance has been helpful to distinguish cardiac involvement from AFD from other common disease entities such as hypertensive heart disease, cardiac amyloidosis, and HCM. The RV may be involved as well.7 The histological basis for hyperenhancement in patients with AFD-related myocardial disease has been shown to be collagen deposition.8 CMR-based LV mass calculation is ideally suited to follow therapeutic response in this and other rare diseases given high reproducibility and has been used to demonstrate regression of LVH in patients with AFD started on enzyme replacement therapy.9
CASE 5 Iron Overload
A 59-year-old woman who had received lifelong transfusions for myelodysplastic syndrome (MDS) presented to her hematologist with progressive DOE. She also had lower extremity edema that was becoming less responsive to oral diuretics. Medications at the time of cardiac evaluation included intermittent chelation therapy with IV desferrioxamine for known hepatic iron overload. Echocardiography demonstrated significantly reduced LV systolic function. Serum chemistries included a markedly elevated brain natriuretic peptide level of 1866 pg/mL (normal: 0-100 pg/mL). Given new-onset heart failure and transfusion history, CMR examination was ordered for the possibility of iron overload as the etiology of cardiomyopathy.
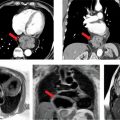
Cine imaging showed severe LV systolic dysfunction with a calculated EF of 14% (Figure 6-10, Video 6-5). Right ventricular systolic function was also severely reduced. T2* mapping was performed using a breath-hold multiecho gradient-echo sequence validated against direct quantification of tissue iron content.10 With increasing echo times (Video 6-6), there was rapid loss of signal intensity in the liver and myocardium indicating significant T2* shortening due to iron deposition in both organs. Pixel-wise fitting of signal intensity vs increasing echo time yielded a myocardial T2* value of 6 milliseconds and hepatic T2* value of 2.5 milliseconds, consistent with severe myocardial and hepatic iron overload.
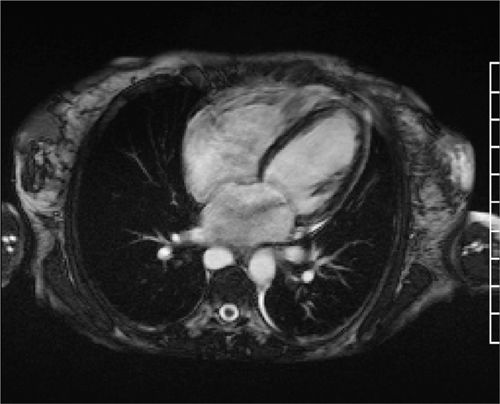
FIGURE 6-10 Systolic frame from a horizontal long-axis SSFP cine acquisition in a patient with new-onset heart failure in the setting of lifelong blood transfusions for myelodysplastic syndrome shows severe biventricular systolic dysfunction. Also note a jet of mitral regurgitation seen as a signal void in the left atrium.
Discussion
Iron overload may result from primary genetic disorders such as hereditary hemochromatosis (HH), or it may occur secondary to exogenous iron administration such as chronic transfusion for patients with thalassemia or other chronic anemias. Hereditary hemochromatosis refers to a group of 4 autosomal disorders, Types 1-3 autosomal recessive and Type 4 autosomal dominant, where mutations in genes encoding iron metabolism proteins lead to excess duodenal iron absorption and subsequent tissue iron accumulation.11 Cardiac iron overload is most common in Type 2, with heart failure deaths common before the age of 30.12 Noninvasive assessment of myocardial iron by CMR is used for screening of high-risk populations, to guide iron chelation therapy and for diagnostic assessment in patients with cardiomyopathy of unknown cause.13 Screening for myocardial iron overload with CMR affords intensification of chelation before iron overload cardiomyopathy is irreversible, fundamentally altering the natural history of conditions that lead to myocardial iron deposition. Introduction of CMR as a routine part of care for patients with thalassemia has reduced deaths due to iron overload by 71%.14
The measurement is highly reproducible across scanners and easily quantifiable.15 Tissue biopsy is unnecessary for iron quantification and may be less reliable given susceptibility to sampling error.10,16 Myocardial T2* at 1.5 Tesla is considered normal if greater than 20 milliseconds; progressively shorter T2* portends worse prognosis.
CASE 6 Friedreich Ataxia
A 42-year-old man with Friedreich ataxia (FA) presented for cardiovascular assessment as part of a longitudinal study of cardiac structure and function in this disorder. He had no family history of FA, but had undergone genetic testing based on concerning neurologic symptoms. Friedreich ataxia is characterized by a loss of function mutations in the frataxin gene on chromosome 9q13.17,18 This patient had expanded trinucleotide (GAA) repeats in the frataxin gene of approximately 600 and 800 repeats in the 2 affected alleles. He was nonambulatory but maintained a regular supine bicycle exercise routine. He adhered to a healthy diet with almost no refined sugar or saturated fat intake given the risk of diabetes in patients with FA. Despite this healthy lifestyle, laboratory testing was notable for low levels of high-density lipoprotein (HDL 31 mg/dL).
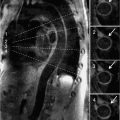
Cardiac magnetic resonance examination with adenosine was performed to assess LV geometry and myocardial perfusion. Cine imaging showed normal LV systolic function and LV mass, but significantly increased relative wall thickness computed as the sum of septal thickness and posterior wall thickness divided by LV end-diastolic dimension (Figure 6-11). Vasodilator stress perfusion imaging revealed diffuse subendocardial hypoperfusion that returned to normal at rest, and late post-gadolinium imaging showed mid-wall fibrosis (Figure 6-12). Quantification of signal intensity vs time curves from endocardium and epicardium at rest and during adenosine indicated a significantly reduced endocardial to epicardial myocardial perfusion reserve index (MPRI) of 0.36.19 Together, these findings were consistent with microvascular disease, possibly exacerbated by features of the metabolic syndrome as indicated by the low HDL. Though asymptomatic from a cardiac standpoint, findings of subclinical myocardial disease (stage B cardiomyopathy) prompted initiation of angiotensin converting enzyme inhibitor20 plus niacin therapy.
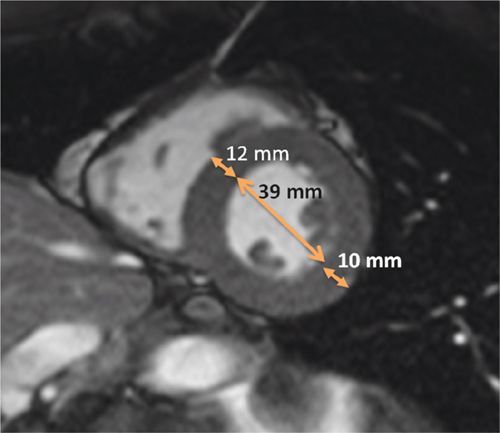
FIGURE 6-11 End-diastolic frame from a mid-short-axis cine acquisition shows increased relative wall thickness (RWT) of 0.56 calculated as: RWT = (septal thickness + posterior wall thickness)/LV internal dimension or (12+10)/39, all measured at end-diastole. Normal RWT for men is less than 0.44. LV mass was normal in this 42-year-old with Friedreich ataxia; thus, increased RWT with normal LV mass indicates concentric remodeling, an abnormal LV geometry with similar increased risk of adverse cardiovascular events as LV hypertrophy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree