INCIDENCE, RISK FACTORS,
AND PREVENTION
1
 INCIDENCE OF CONGENITAL HEART DISEASE
INCIDENCE OF CONGENITAL HEART DISEASE
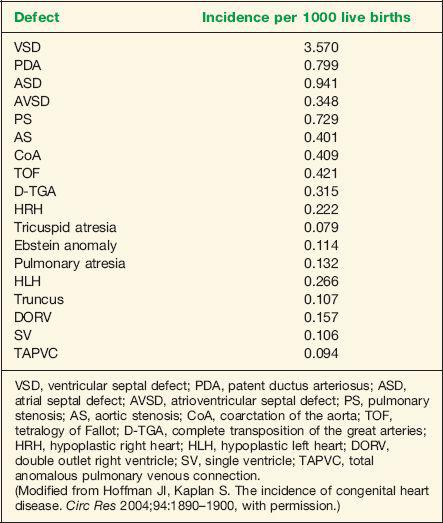
Congenital heart disease (CHD) is the most common severe congenital abnormality (1). Half of the cases of CHD are minor and are easily corrected by surgery, with the remainder accounting for over half of the deaths from congenital abnormalities in childhood (1). Moreover, CHD results in the most costly hospital admissions for birth defects in the United States (2). The incidence of CHD is dependent on the age at which the population is initially screened and the definition of CHD used. Inclusion of a large number of premature neonates in a study may increase the incidence of CHD. Both patent ductus arteriosus and ventricular septal defects are common in premature infants. An incidence of 8 to 9 per 1000 live births has been reported in large population studies (1). Of all cases of CHD, 46% are diagnosed by the first week of life, 88% by the first year of life, and 98% by the fourth year of life (1). The incidence of CHD is also influenced by the inclusion of bicuspid aortic valve, the incidence of which is estimated at 10 to 20 per 1000 live births (3,4). Bicuspid aortic valve may be associated with considerable morbidity and mortality in affected individuals (3). Furthermore, accounting for subtle anomalies such as persistent superior vena cava (5 to 10 per 1000 live births) and isolated aneurysm of the atrial septum (5 to 10 per 1000 live births) results in an overall incidence of CHD approaching 50 per 1000 live births (5). CHD remains the most common severe abnormality in the newborn; its prenatal diagnosis allows for better pregnancy counseling and improved neonatal outcome. Table 1-1 lists incidence by specific type of CHD (6).
 RISK FACTORS FOR CONGENITAL HEART DISEASE
RISK FACTORS FOR CONGENITAL HEART DISEASE
The majority of fetuses with CHD have no known risk factors (7). In spite of this, fetal echocardiography has been traditionally performed on pregnancies with identifiable risk factors for CHD. Risk factors for CHD can be grouped into two main categories: fetal factors and maternal factors (Table 1-2). The association of CHD with a prior family history of such, or in the presence of fetal chromosomal abnormalities, will be discussed in the following chapter.
Fetal Risk Factors
Extracardiac Anatomic Abnormalities
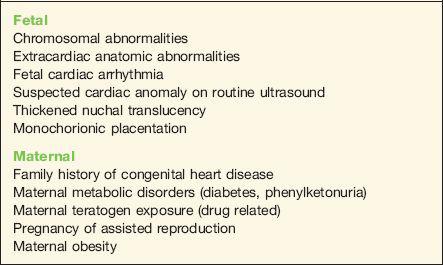
The presence of extracardiac abnormalities in a fetus is frequently associated with CHD and is thus an indication for fetal echocardiography. The risk of CHD is dependent on the specific type of fetal malformation. Abnormalities detected in more than one organ system increase the risk of CHD and also of concomitant chromosomal abnormalities. Nonimmune hydrops in the fetus is frequently associated with CHD. Incidence of abnormal cardiac anatomy is reported in about 10% to 20% of fetuses with nonimmune hydrops (8,9). Table 1-3 lists extracardiac fetal abnormalities and the incidence of associated CHD (10–24).
Fetal Cardiac Arrhythmia
The presence of fetal cardiac rhythm disturbances may be associated with an underlying structural heart disease. Isolated extrasystoles account for more than 90% of fetal cardiac arrhythmias (25). Overall, about 1% of fetal cardiac arrhythmias are associated with CHD (8). Complete heart block, on the other hand, is associated with structural cardiac abnormalities in about 50% of fetuses, with the remaining pregnancies associated with the presence of maternal Sjögren antibodies (26). Diagnosis and management of fetal cardiac rhythm disturbances is discussed in detail in Chapter 25.
Suspected Cardiac Anomaly on Routine Ultrasound
A risk factor with one of the highest yields for CHD is the suspicion for the presence of a cardiac abnormality during routine ultrasound scanning. CHD is confirmed in about 40% to
| TABLE 1-1 | Types and Incidence of Human Congenital Heart Disease |
50% of pregnancies referred with this finding (8,9). In view of this, and the fact that most infants born with CHD are born to pregnancies without risk factors, systemic ultrasound examination of the fetal heart should not be limited to pregnant mothers with known risk factors. The value of routine ultrasound in the screening for CHD is discussed in Chapter 3.
Thickened Nuchal Translucency
Measurement of fetal nuchal translucency (NT) thickness in the late first and early second trimesters of pregnancy is currently established as an effective method for individual risk
| TABLE 1-2 | Risk Factors for Congenital Heart Disease |
| TABLE 1-3 | Incidence of Associated Congenital Heart Disease (CHD) with Extracardiac Malformation |
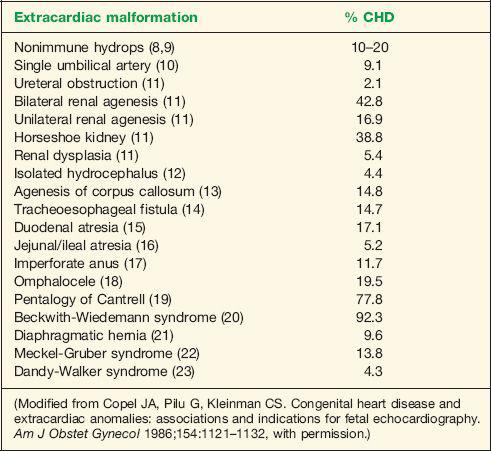
assessment for fetal chromosomal abnormalities. Several reports have noted an association between increased NT and genetic syndromes and major fetal malformations including cardiac defects (27,28). The prevalence of major cardiac defects increases exponentially with fetal NT thickness, without an obvious predilection to a specific type of CHD (29). An NT thickness of greater than or equal to 3.5 mm in a chromosomally normal fetus has been correlated with a prevalence of CHD of 23 per 1000 pregnancies, a rate that is higher than pregnancies with a family history of CHD (29,30). In this setting of an NT that is greater than or equal to 3.5 mm, referral for fetal echocardiography is thus warranted. Finding an NT thickness of greater than or equal to 3.5 mm may lead to an earlier diagnosis of all major types of CHD (31). Table 1-4 lists the prevalence of CHD with NT thickness in chromosomally normal fetuses (30).
Monochorionic Placentation
The incidence of CHD in fetuses of monochorionic placentation is increased (32,33). This increased risk of CHD is noted even after excluding cardiac effects of twin–twin transfusion syndrome (TTTS) (32). In a cohort study of 165 sets of monochorionic twins, the overall risk of at least one of a twin pair having a structural CHD was 9.1% (32). This risk was 7% for monochorionic–diamniotic twins and 57.1% for at least one twin member of monochorionic–monoamniotic twins (32). If one twin member is affected, the risk that the other twin member is also affected is 26.7% (32). A systemic literature review of 830 fetuses from monochorionic–diamniotic twin pregnancies confirms an increased risk for CHD independent of TTTS (33). Ventricular septal defects were the most common type of CHD in non-TTTS fetuses, and pulmonary stenosis and atrial septal defects were significantly more prevalent in fetuses of pregnancies complicated with TTTS (33).
Maternal Risk Factors
Maternal Metabolic Disease
Maternal metabolic disorders, mainly diabetes mellitus, have a significant effect on the incidence of CHD. The incidence of CHD is fivefold higher in infants of diabetic mothers when
| TABLE 1-4 | Prevalence of Congenital Heart Disease (CHD) with Nuchal Translucency Thickness in Chromosomally Normal Fetus |
compared to controls (34). Ventricular septal defects and transposition of the great arteries are common cardiac defects in fetuses of diabetic pregnancies (34). Poor glycemic control in the first trimester of gestation, as evidenced by an elevated glycohemoglobin level (HgA1c), has been strongly correlated with an increased risk of structural defects in infants of diabetic mothers (35,36). Although some studies have identified a level of glycohemoglobin above which the risk for fetal structural abnormalities is increased (35), other studies have failed to identify a critical level of glycohemoglobin providing an optimal predictive power for CHD screening (37). Fetal echocardiography should therefore be offered to all pregnancies with initial glycohemoglobin levels above the upper limits of normal (37).
Another metabolic disorder that is associated with CHD is phenylketonuria. Women with phenylketonuria should be aware of the association of fetal CHD with elevated maternal phenylalanine levels (38). This is particularly important as phenylketonurics usually follow unrestricted dietary regimens in adulthood. Fetal exposure during organogenesis to maternal phenylalanine levels exceeding 15 mg/dL is associated with a 10- to 15-fold increase in congenital heart disease (39). Other fetal abnormalities in phenylketonurics include microcephaly and growth restriction (38). In the presence of maternal metabolic disease, preconception counseling and tight metabolic control immediately prior to and during organogenesis is required in order to reduce the incidence of fetal CHD.
Maternal Teratogen Exposure (Drug-related Congenital Heart Disease)
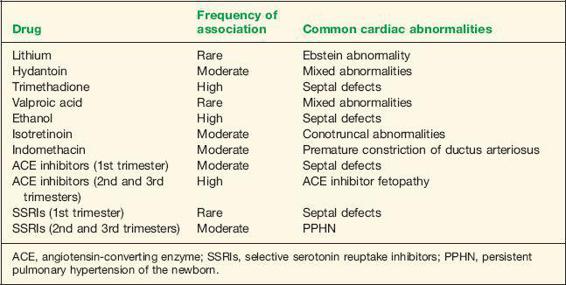
The effects of maternal exposure to drugs during cardiogenesis has been widely studied. Numerous drugs have been implicated as cardiac teratogens. Evidence suggests that the overall contribution of teratogens to CHD is small (40). Available literature suggests that maternal use of lithium, anticonvulsants, ethanol, isotretinoin, indomethacin, angiotensin-converting enzyme (ACE) inhibitors, and selective serotonin reuptake inhibitors may increase the risk of cardiovascular abnormalities in the newborn (Table 1-5).
Initial retrospective reports regarding the teratogenic risk of lithium treatment in pregnancy showed a strong association between lithium use and Ebstein anomaly in the fetus (41). More recent controlled studies, however, have consistently reported a lower risk for CHD in exposed fetuses. Four case-control studies of Ebstein anomaly involving a total of 208 affected children found no association with maternal lithium intake in pregnancy (42–45). A cohort study on the effect of lithium exposure in pregnancy showed no significant risk to the fetus (46). These findings suggest that the teratogenic risk of lithium exposure is lower than previously reported, and that the risk/benefit ratio of prescribing lithium in pregnancy should be evaluated in light of this modified risk estimate.
Anticonvulsants, a class of drugs that includes phenytoin and sodium valproate, are commonly used in the treatment of epilepsy or pain management in pregnancy. An incidence of congenital defects varying from 2.2% to 26.1% has been noted in pregnancies exposed to phenytoin (47). Some evidence suggests that the teratogenic effect of phenytoin is related to elevated amniotic fluid levels of oxidative metabolites secondary to low activity of the clearing enzyme epoxide hydrolase (48). A fetal hydantoin syndrome consisting of variable degrees of
| TABLE 1-5 | Drug-related Congenital Heart Disease |
hypoplasia and ossification of distal phalanges and craniofacial abnormalities has been described (49). CHD is often observed in conjunction with this syndrome (50). Trimethadione, an anticonvulsant primarily used in the treatment of petit mal seizures, is associated with a high incidence of congenital defects. Defects include craniofacial deformities, growth abnormalities, mental retardation, limb abnormalities, and genitourinary abnormalities (51). Cardiac abnormalities are common, with septal defects occurring in about 20% of exposed fetuses (51). Valproic acid has also been associated with congenital defects, with the most serious abnormality being neural tube defects (1% to 2%) (52). Although some reports have suggested an increased risk of CHD in fetuses exposed to valproic acid (53), others could not establish a causal relationship (52,54).
The fetal alcohol syndrome, consisting of facial abnormalities, growth restriction, mental retardation, and cardiac abnormalities, has been well described in women consuming heavy amounts of alcohol in pregnancy (55). Cardioteratogenic effects of ethanol in the chick embryo have been confirmed in concentrations comparable to human blood alcohol levels (56). CHD has been identified in 25% to 30% of infants with fetal alcohol syndrome, with septal defects representing the most common lesions (55,57).
Isotretinoin is a vitamin A derivative prescribed for the treatment of severe cystic acne. Since its introduction, several reports have appeared in the literature describing the teratogenic effect of this medication. A characteristic pattern of malformations is observed, which includes central nervous system, craniofacial, branchial arch, and cardiovascular abnormalities (58). Cardiac abnormalities are usually conotruncal in origin (59,60). The mechanism of teratogenicity is probably related to free radical generation by metabolism with prostaglandin synthase (61).
Indomethacin, a nonsteroidal anti-inflammatory drug, is used in the treatment of preterm labor. In the fetus, indomethacin therapy may lead to premature constriction of the ductus arterior (see Chapter 13 for details). Several neonatal complications, which appear to be limited to indomethacin exposure beyond 32 weeks of gestation, include oliguria, necrotizing enterocolitis, and intracranial hemorrhage (62). Cardiovascular complications include a higher risk for patent ductus arteriosus requiring surgical ligation in indomethacin-exposed infants (62).
ACE inhibitors are commonly used antihypertensive medications. Fetal exposure to ACE inhibitors in the first trimester of pregnancy has been associated with an increased risk of major congenital malformation that was 2.7 times greater than the background risk or the risk of fetuses exposed to other antihypertensive medications (63). The increase in major malformations primarily affects the cardiovascular (risk ratio, 3.72) and central nervous systems (risk ratio, 4.39) (63). Atrial and ventricular septal defects represent the most common cardiac abnormalities (63). Fetal exposure to ACE inhibitors in the second and third trimesters of pregnancy is associated with “ACE inhibitor fetopathy,” which includes oligohydramnios, intrauterine growth restriction, hypocalvaria, renal failure, and death (64).
Selective serotonin reuptake inhibitors (SSRIs) represent a new class of antidepressants that has gained wide acceptance for the treatment of depression and anxiety during pregnancy (65). Specific SSRI medications include citalopram (Celexa), fluoxetine (Prozac), paroxetine (Paxil), and sertraline (Zoloft). Pregnancies exposed to SSRIs in the first trimester have shown an increased risk of congenital heart defects (66–68). Paroxetine has been singled out as the SSRI with the greatest association with congenital heart malformations, primarily atrial and ventricular septal defects (68). A meta-analysis of seven studies noted a significant overall increased risk of 74% for cardiac malformations in women exposed to paroxetine in the first trimester of pregnancy (69). The U.S. Food and Drug Administration, Health Canada, and the drug manufacturer issued a warning in 2005 to health care professionals regarding the potential risk to infants born to mothers receiving paroxetine during the first trimester of pregnancy (70). Two recent large controlled studies, however, do not support the association of overall SSRI exposure in the first trimester of pregnancy with an increased risk of congenital heart defects or of most other categories of birth defects (71,72). Individual SSRIs may confer increased risks for some specific defects, but it should be recognized that the specific defects implicated are rare and the absolute risks are small (71,72).
SSRI exposure after the 20th week of gestation has been associated with an increased risk of persistent pulmonary hypertension of the newborn (PPHN) (73). PPHN occurs in 1 to 2 per 1000 live births and is associated with increased morbidity and mortality. SSRI exposure increases this risk to about 6 to 12 per 1000 neonates, a sixfold increase over the background risk (73). Possible mechanisms of action include an accumulation of serotonin in the lung in exposed fetuses (74). Serotonin has vasoconstrictor properties and a mitogenic effect on pulmonary smooth muscle cells, which may result in the proliferation of smooth muscle cells, the characteristic histologic pattern in PPHN (75,76).
Pending further studies, SSRI use during pregnancy should be individualized. Health care workers and their patients must weigh both the benefits and the potential risks of SSRI treatment in the context of the risk of recurrence of depression if maintenance therapy is discontinued.
Pregnancies of Assisted Reproductive Technology
Infants born to pregnancies of assisted reproductive technology are more likely to be born preterm, of low birth weight, and small for gestational age (77). This increased neonatal morbidity applies to multiple and singleton births (78). The evidence relating to the risk of birth defects is somewhat less clear. A report of systematically reviewed and pooled epidemiologic data assessing the risk of birth defects suggests a 30% to 40 % increase following assisted reproductive technologies (in vitro fertilization [IVF] and/or intracytoplasmic sperm injection [ICSI]) (79). Another population-based study on congenital malformations in children born after IVF with matched controls noted a fourfold increase in CHD in the IVF population, with the majority of cardiac anomalies representing atrial and ventricular septal defects (80). This same rate of fourfold increase in CHD was also noted in pregnancies conceived through ICSI (81).
Maternal Obesity
The prevalence of obesity, which is defined as a body mass index (BMI) greater than or equal to 30 kg/m2, is increasing at an exponential rate. An established association between neural tube defects and prepregnancy maternal obesity exists (82). Several studies have noted an increased risk of congenital heart defects in obese pregnant mothers when compared to average-weight mothers (83,84). This increased risk is relatively small: 1.18-fold for the obese mother and 1.40-fold for the morbidly obese mother (BMI >35 kg/m2). Atrial and ventricular septal defects contribute to the majority of this increased risk (84).
 PREVENTION OF CONGENITAL HEART DISEASE
PREVENTION OF CONGENITAL HEART DISEASE
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree