Subha V. Raman, MD, MSEE, FACC, FAHA
INTRODUCTION
Ischemic heart disease (IHD) requires precise assessment of the myocardium—the presence and extent of irreversibly injured, ischemic and nonischemic/noninjured muscle are essential characteristics that inform diagnosis, treatment planning and prognosis. Cardiac magnetic resonance (CMR) and, the more recent, cardiac computed tomography (CCT) offer considerable improvement in accurate characterization of ischemic myocardial disease over traditional modalities. Both CMR and CCT afford greater spatial resolution which has implications for detecting thin but viable myocardium as well as nontransmural infarct scar. Modalities with lower spatial resolution like single photon emission computed tomography (SPECT) may erroneously deem nonviable regions of myocardial thinning; appropriate revascularization may thereby not be performed in the patient who needs it to improve cardiac function. CMR can be done without ionizing radiation and consistently outperforms SPECT in ischemia detection, with fewer false-positive and false-negative results. CMR’s ability to offer ischemia detection combined with scar delineation in the setting of complex revascularization histories and equivocal symptoms makes it a preferred modality for patients of ischemic myocardial disease.
The cornerstone techniques for assessing ischemia and viability are first-pass perfusion imaging and LGE, respectively. Further, perfusion reserve or ischemia may be evaluated using pharmacologic or physiologic maneuvers. Typical pharmacological agents used with CMR perfusion imaging include vasodilators like adenosine, dipyridamole, and regadenoson. Ischemia may also be assessed with cine imaging and positive inotropic drugs like dobutamine. Physiologic stress protocols include supine bicycle ergometry and treadmill exercise, with cine and/or perfusion imaging performed at rest and after peak exertional stress. For completeness, we mention cardiac magnetic resonance spectroscopy (CMRS) to measure, for instance, the ratio of phosphocreatine to adenosine triphosphate (ATP) at rest and over several minutes of handgrip exercise or dobutamine infusion. The considerably lower biologic availability of phosphorus for the MR signal relative to hydrogen, the species imaged in the vast majority of clinical CMR exams worldwide, requires specialized approaches for measurement. However, in centers adept at these approaches, CMRS affords direct, noninvasive assessment of various aspects of myocardial metabolism not feasible with any other in vivo imaging technology. Ongoing technical advances such as hyperpolarized contrast agents allow imaging of enzymatic processes and exciting windows into future possibilities.
CASE 1 Adenosine Stress CMR and Epicardial Coronary Artery Disease
A 51-year-old man has a history of coronary artery disease (CAD). Post bypass surgery (CABG) 6 years ago and a percutaneous stent placement 9 years ago, he presented with chest pain radiating to the jaw and accompanied by nausea and diaphoresis. Coronary angiography 6 months prior to this presentation had demonstrated serial left anterior descending (LAD) disease with patent left internal mammary artery (LIMA) conduit to distal LAD, occluded left circumflex (LCX) and right coronary (RCA) arteries, and patent saphenous vein graft (SVG) to posterior descending artery (PDA). In addition, there was a 90% stenosis in an SVG to sequential lateral wall targets for which percutaneous stent placement was performed. Serial troponin measurements were negative for myocardial injury, and electrocardiography (ECG) was unchanged from the prior admission. To determine the presence and extent of ischemia plus scar in the setting of multiple prior interventions and a potential target for subsequent revascularization, he was referred for pharmacological stress CMR.
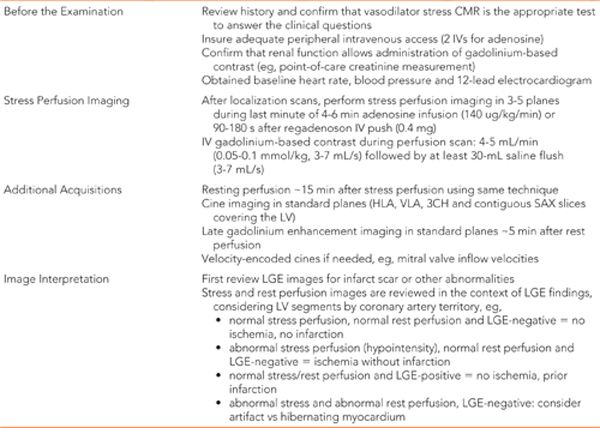
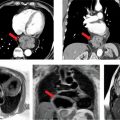
Late gadolinium enhancement imaging showed no scar—myocardium was viable in all segments (Figure 3-1). Perfusion during infusion of adenosine showed inferior and inferoseptal hypoperfusion not present at rest (Figure 3-2, Videos 3-1 and 3-2). Cine imaging revealed inferior wall motion abnormalities (Figure 3-3, Videos 3-3 and 3-4).
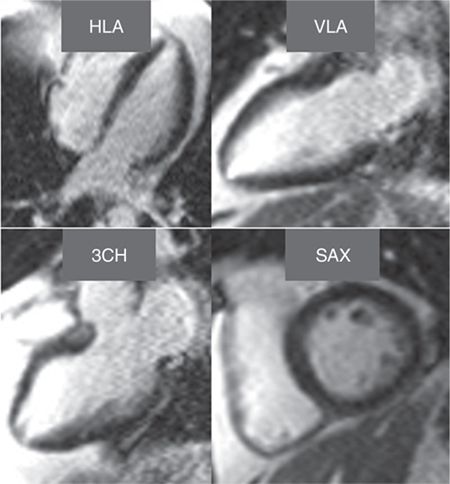
FIGURE 3-1 Late gadolinium enhancement imaging is shown in horizontal long-axis (HLA), vertical long-axis (VLA), 3-chamber (3 CH) and mid-short-axis (SAX) planes. No infarct scar is demonstrated, consistent with viability in all coronary distributions.
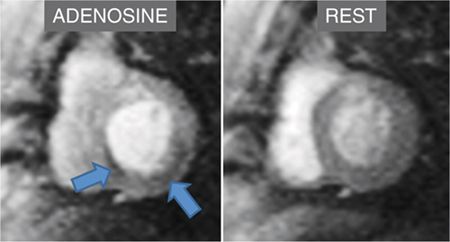
FIGURE 3-2 First-pass myocardial perfusion imaging during adenosine infusion demonstrates inferior and inferoseptal abnormalities (arrows) not present at rest.
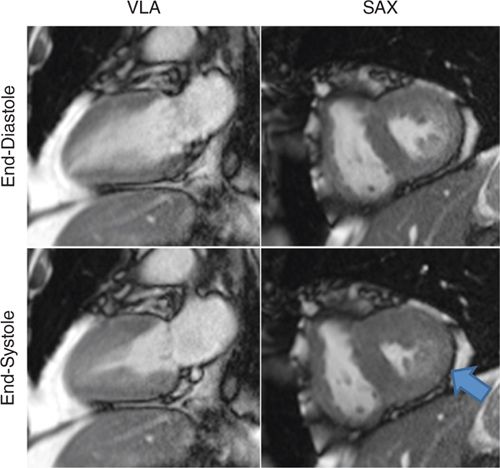
FIGURE 3-3 Frames from cine imaging demonstrate inferior wall motion abnormalities, evident as abnormal contraction at end-systole vs end-diastole in both vertical long-axis (VLA) and mid-short-axis (SAX) planes.
CMR findings of ischemia in the setting of symptoms of angina prompted return to the catheterization laboratory that demonstrated interim development of serial stenosis in the SVG to PDA (Figure 3-4), consistent with the inferior perfusion abnormality demonstrated during adenosine stress. He underwent successful percutaneous revascularization of these lesions with relief of symptoms.
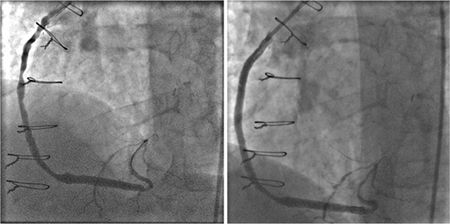
FIGURE 3-4 Invasive angiography of a saphenous vein graft to the posterior descending coronary artery shows serial high-grade stenoses (left panel) that are relieved with percutaneous angioplasty and stent placement (right panel).
CASE 2 Adenosine Stress CMR and Microvascular Disease
A 70-year-old overweight woman (BMI 28.1 kg/m2) with hypertension and hyperlipidemia was seen by a cardiologist for intermittent substernal chest pain. Coronary angiography 1 year prior to this evaluation showed no obstructive epicardial CAD. Laboratory testing indicated low high-density lipoprotein level (45 mg/dL) and elevated triglycerides (218 mg/dL); low density lipoprotein (LDL) level was 90 mg/dL on atorvastatin therapy. Adenosine vasodilator stress CMR was ordered to determine the presence and extent of myocardial ischemia as the cause of chest pain.
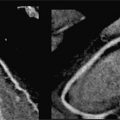
Resting systolic function was normal, but perfusion imaging during adenosine infusion revealed diffuse subendocardial abnormality (Figure 3-5). Late gadolinium enhancement imaging revealed no infarct scar or infiltrate. In light of the CMR findings, the patient was given a diagnosis of microvascular disease and initiated on long-acting nitroglycerin with symptomatic relief. In addition, her lipid-lowering regimen was intensified along with addition of daily aspirin intake. A regular exercise program was initiated as well.
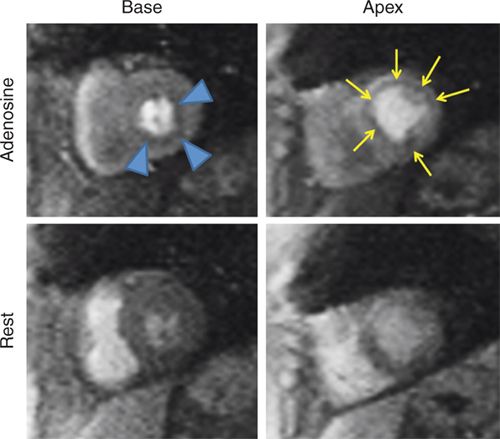
FIGURE 3-5 Frames from first-pass perfusion imaging acquired in basal and apical short-axis planes are shown. The upper row images were acquired during adenosine infusion, and the lower images 15 minutes later. There is diffuse subendocardial perfusion abnormality with adenosine (arrowheads and arrows) in both the basal and apical slices. In this patient without significant epicardial coronary artery obstruction, this pattern is consistent with microvascular disease.
VASODILATOR STRESS CMR PROTOCOL
Knowing the pulse sequences available for perfusion, cine, and LGE imaging (Chapter 1), one can now bring together these techniques in a clinical stress perfusion CMR protocol. Adenosine produces augmented blood flow downstream from coronary arteries of normal luminal diameter, while the arterial tree downstream from significantly stenotic coronary segments have already recruited whatever circulatory reserve is available. Thus, perfusion imaging during adenosine infusion demonstrates reduced enhancement in myocardium supplied by stenotic vs nonstenotic coronary arteries. Adenosine should be avoided in patients with hypotension, high-degree atrioventricular (AV) block, significant obstructive lung disease and those whose current medications include dipyridamole. The literature also supports avoidance of caffeine up to 48 hours prior to adenosine stress perfusion examination. It should be noted that many CMR laboratories are switching from adenosine (or even the older agent dipyridamole) to the newer agent regadenoson for vasodilator stress. The latter is a selective A2A adenosine receptor agonist that typically does not produce AV blocking or bronchoconstrictive effects.1 A typical vasodilator stress CMR protocol is shown in Table 3-1 (adapted from Kramer et al2). Data interpretation may be qualitative or quantitative. In clinical practice, visual analysis is preferred though increasing availability of robust, in-line quantitative tools that may change this paradigm.
The early work of Schaefer et al3 and Hartnel et al4 demonstrated the use of dipyridamole in vasodilator perfusion CMR in patients with a single slice, thus not visualizing the whole myocardium. Subsequent studies using multislice perfusion imaging showed comparative effectiveness vs radionuclide imaging5,6 and superior diagnostic accuracy in identifying CAD in patients undergoing invasive coronary angiography.7,8 A limitation of using coronary angiography as a reference standard is its inability to determine the physiological extent of ischemia. Studies using fractional flow reserve measurement lend credence to the ability of vasodilator stress perfusion CMR to identify hemodynamically significant epicardial CAD.9
The optimal contrast dose was studied in the multicenter MR-IMPACT study where investigators used varying contrast dose in five dose ranges from 0.01 to 0.1 mmol/kg in patients to detect CAD as against coronary angiography.10 Although a dose of 0.1 mmol/kg was found to have a superior diagnostic performance in detecting CAD compared to low dose, it fared equally well in terms of accuracy vs SPECT, thus endorsing stress perfusion CMR as a suitable alternative to radiation requiring nuclear imaging.
The American College of Cardiology currently recommends the use of pharmacological stress cardiac imaging tests for detection of CAD in symptomatic patients who have uninterpretable ECG or contraindications to exercise stress test with an intermediate pretest probability.11 In a study by Vogel-Clussen et al, this approach was shown to be superior to SPECT imaging in identifying the etiology of chest pain in 27 patients who presented to emergency room having an intermediate-level likelihood of CAD.12 Subendocardial ischemia that was missed on SPECT was identified on cardiac MRI, suggesting microvascular disease. They were also found to have higher readmission rates compared to normal CMR cohort, thus conferring prognostic value. Data from clinical evaluation of Magnetic Resonance Imaging in coronary heart disease (CE-MARC) study has shown consolidative proof of the advantage CMR perfusion offers over SPECT with improved sensitivity (86.5% vs 66.5%, p<0.0001) and a negative predictive value (90.5% vs 79.1%, p<0.0001).13 In conclusion, vasodilator stress test has become increasingly appealing to the clinician due to its ability to detect perfusion defects at the sub-endocardial level.
CASE 3 Dobutamine Stress CMR and Epicardial Coronary Artery Disease
An 80-year-old woman with previous right coronary artery percutaneous intervention presented with dyspnea and chest pressure to her primary care physician. In view of these symptoms, she was transferred to a tertiary care hospital for further evaluation. Serial troponin measurements were negative for myocardial injury. With hematoma and bleeding at the catheterization site during her most recent PCI, she was reluctant to undergo repeat invasive angiography so instead was referred for CMR with dobutamine stress to determine the presence and extent of ischemia.
Resting CMR images revealed inferior hypokinesis with overall preserved LV systolic function. With dobutamine stress and atropine to achieve the target heart rate (HR), the patient developed chest pain that resolved with termination of the infusion. Extensive stress induced inferior wall motion abnormality that persists in recovery was noted along with a perfusion deficit not seen at rest consistent with RCA territory ischemia. LGE imaging showed no evident scar in this or any other myocar-dial region (Figures 3-6 to 3-8, and Videos 3-5 and 3-6). Together with stress wall motion and perfusion abnormalities not present at rest, these findings indicated ischemic but viable myocardium.
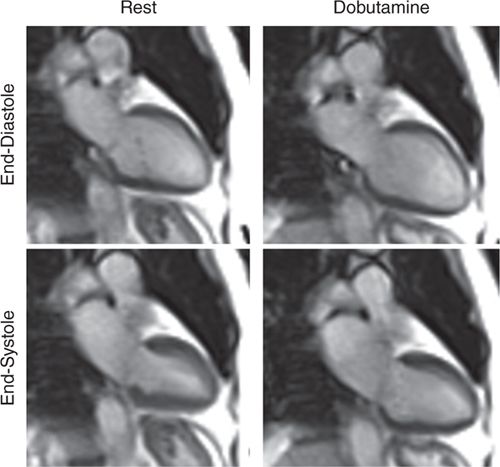
FIGURE 3-6 End-diastolic and end-systolic frames obtained at rest and at peak dobutamine infusion plus atropine to achieve target heart rate show a mild resting inferior wall motion abnormality that becomes considerably worse with inotropic stress.
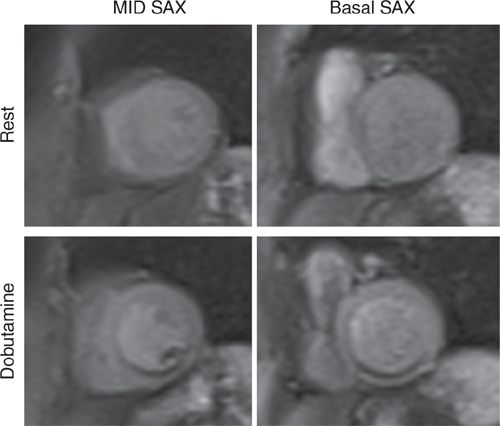
FIGURE 3-7 First-pass perfusion imaging at rest (top row) and during peak dobutamine infusion (bottom row) is shown in mid-short-axis (left) and basal short-axis (right) planes. There is a large, dense inferior perfusion defect with dobutamine not present at rest.
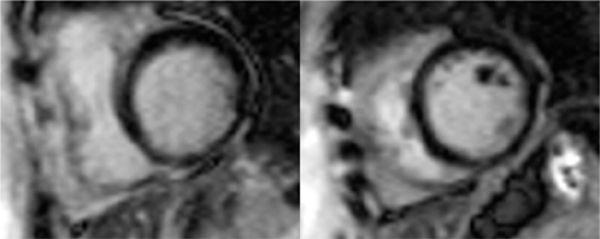
FIGURE 3-8 Late gadolinium enhancement imaging in basal short-axis (left) and mid-short-axis (right) planes shows no significant infarct scar. Together with stress wall motion and perfusion abnormalities not present at rest, these findings indicate ischemic but viable myocardium.
In light of stress CMR indicating likely re-occlusion of the RCA with a stent, resolution of symptoms with titration of medications, and aversion to undergo repeat intervention, medical management was continued without repeat invasive angiography. At the 6-month follow-up, the patient reported resumption of activities of daily living without limiting symptoms.
CASE 4 Dobutamine Stress CMR and Microvascular Disease
A 25-year-old African-American man with lupus was initially seen in the outpatient rheumatology clinic for worsening rash. He had been diagnosed with discoid lupus 3 years earlier, and 1 week prior to admission was started on pulse steroids due to increase in rash. Physical examination demonstrated weight loss over the last week, HR 100, BP 90/60, and a diffuse, erythematous and pruritic rash with blistering and oozing. Because of progressive rash, weight loss, a generalized ill appearance, he was diagnosed with lupus flare and admitted for intravenous fluids, nutritional support and more intensive therapy. On admission, he was noted to have a room air oxygen saturation of 89%. Empiric treatment for pneumonia was initiated, but dyspnea persisted and was worse with even minimal exertion. Serial electrocardiograms were obtained that showed dynamic T-wave inversion (Figure 3-9) concerning for myocardial ischemia. To assess for myocardial ischemia vs myocardial injury in the setting of lupus flare, CMR with dobutamine stress was performed.
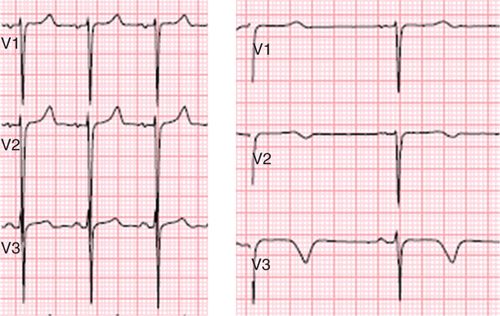
FIGURE 3-9 Dynamic T-wave inversion across the precordial leads was noted between an electrocardiogram obtained at the time of admission (left) and an ECG obtained several days later during an episode of dyspnea (right) in a patient with lupus flare.
Rest CMR images showed normal LV wall motion. With graded dobutamine infusion, cine imaging demonstrated extensive wall motion abnormalities not present at rest (Figures 3-10 and 3-11, Videos 3-7 and 3-8).
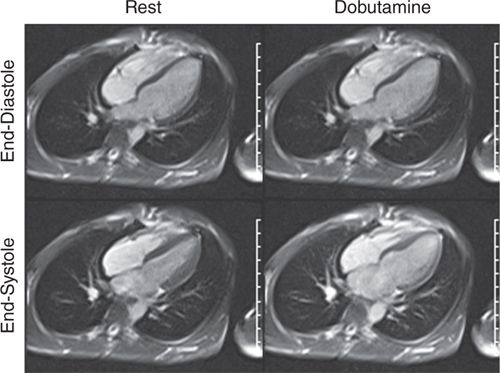
FIGURE 3-10 End-diastolic and end-systolic frames of a horizontal long-axis cine acquisition show normal contraction of all LV segments at rest. The same frames obtained at peak dobutamine infusion show minimal thickening of LV myocardium at end-systole, suggesting diffuse wall motion abnormality due to ischemia.
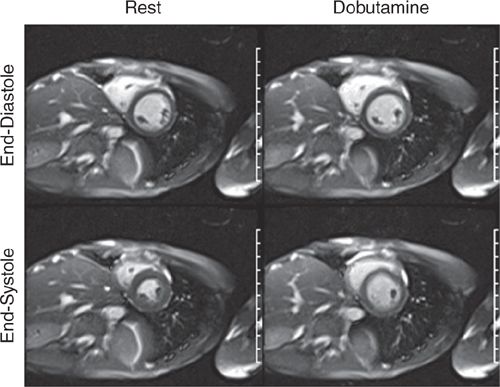
FIGURE 3-11 End-diastolic and end-systolic frames of a mid-short-axis cine acquisition show normal contraction of all LV segments at rest. The same frames obtained at peak dobutamine infusion show minimal thickening of LV myocardium at end-systole, suggesting diffuse wall motion abnormality due to ischemia.
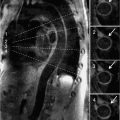
Perfusion imaging showed diffuse subendocardial abnormality at peak dobutamine infusion, and late post-gadolinium enhancement imaging showed no evident myocardial scar or infiltrate (Figure 3-12). Wall motion returned to normal with termination of dobutamine infusion and administration of intravenous metoprolol. Given diffuse ischemia, the patient was referred for invasive angiography that showed normal coronary arteries (Figure 3-13). These findings together with diffuse subendocardial perfusion abnormality and global LV dysfunction with stress were felt to be most consistent with severe microvascular disease—possibly a small vessel vascuilitis in the setting of lupus flare. With high-dose intravenous steroids, both the presenting rash and exertional dyspnea gradually improved.
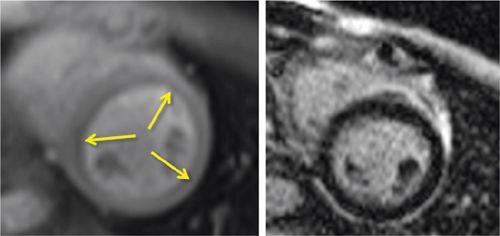
FIGURE 3-12 Stress perfusion (Left) obtained at peak dobutamine infusion showed diffuse subendocardial hypoperfusion (arrows). Late post-gadolinium imaging (right) demonstrated no significant myocardial infarct scar. Together, these findings were consistent with diffuse subendocardial ischemia, ie, microvascular disease.
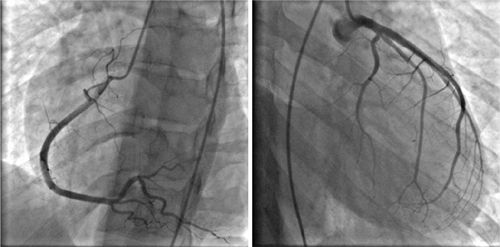
FIGURE 3-13 Invasive angiography was performed in a 25-year-old man with discoid lupus and dyspnea on exertion. All epicardial branches of the right and left coronary arteries were angiographically normal, suggesting that diffuse subendocardial ischemia by stress CMR was indicative of microvascular disease.
DOBUTAMINE STRESS CMR PROTOCOL
Dobutamine is a positive inotropic agent that increases HR and contractility. The typical question for dobutamine stress imaging tests is whether or not LV wall segments increase in contractility in response to intravenous dobutamine infusion. A segment with adequate coronary flow reserve (CFR) will demonstrate increased contractility but one with limited CFR (such as that supplied by a stenotic epicardial coronary artery) will not be able to increase in contractility and may become hypokinetic or akinetic. Dobutamine stress protocols are closer to “physiologic” stress than vasodilator infusion, and atropine can be used in addition to dobutamine to achieve target HR determined by age. Dobutamine has been used for many years in pharmacologic stress echocardiography.14 Similarly, dobutamine stress CMR fundamentally involves repeated sets of cine imaging performed at increasing doses of dobutamine infusion until a suitable endpoint is reached.
Both the ordering and performing physicians are responsible for insuring that the correct test is being done to answer the clinical questions for a given patient. Conditions that are considered contraindications to dobutamine stress include uncontrolled hypertension, severe aortic valve stenosis, unstable angina, hypertrophic cardiomyopathy with outflow tract obstruction, uncontrolled arryhthmia, pericarditis, myocarditis, and endocarditis.2 Atropine should not be given in addition to dobutamine in patients with narrow-angle glaucoma, obstructive uropathy, and obstructive gastrointestinal disorders. As with any exogenous drug or contrast agent, known hypersensitivity to these agents should preclude repeat exposure. Table 3-2 details the typical protocol for dobutamine stress CMR. Increasing doses of dobutamine are infused up to a maximum dose of 40 ug/kg/min, unless one of the following endpoints is reached first: target heart rate (0.85*[220-age]), new or worsening wall motion, complex cardiac arrhythmia, severe hypertension (eg, systolic blood pressure >200 mm Hg) or drop in SBP of 20 mm Hg from baseline, severe symptoms such as angina, dyspnea or patient request.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree