CHAPTER 9 Liver Metastases
MODALITY CHOICES AND TECHNIQUES
Computed Tomography
Generally, most metastases (any primary) are hypovascular and are best depicted on PV phase CT. Metastases that are considered hypervascular include renal cell carcinoma, carcinoid, neuroendocrine tumors, thyroid carcinoma, and melanoma and have been shown to be better seen on HA phase imaging. Breast carcinoma hepatic metastases can be hypervascular, but these represent a small minority of breast cancer liver metastases.
Triple-phase technique is desirable at initial imaging (referral for staging or clinical suspicion of liver metastases) to accurately characterize any lesions, benign or malignant, which may be encountered. However, follow-up of patients with known liver metastases with PV phase enhanced CT alone is generally sufficient and will avoid unnecessary radiation exposure. Support for this approach comes from work by Frederick and colleagues, who looked retrospectively at a series of 84 consecutive triple-phase CT scans in 80 women who were known or suspected to have breast cancer liver metastases.1 They evaluated each phase independently for focal liver lesions and found that the PV phase outperformed noncontrast and HA phases for lesion detection and identified more malignant lesions than the other phases. Importantly, no CT interpretation was converted from negative to positive for the presence of liver lesions based on the addition of either noncontrast or HA phase images. The authors noted that noncontrast and HA phase imaging added additional information in some cases, but found it difficult to justify their routine use when the clinical question was the presence or absence of metastatic disease.
The same group subsequently published a larger, prospective study of liver metastases detection in 300 consecutive triple-phase helical CT examinations in breast cancer patients.2 PV phase images were reviewed alone for the number and size of focal liver lesions. PV images were then reviewed in conjunction with noncontrast images, and again in conjunction with HA phase images. Little improvement in sensitivity for lesion detection was noted when comparing PV phase alone with PV phase in conjunction with noncontrast imaging (97% to 100%) or HA phase (96% to 98%), suggesting little additional benefit was derived from the addition of noncontrast or HA phases. Two to 4% of lesions were seen on HA phase imaging only. These represented either false-positive cases or were seen in conjunction with other metastases on the PV phase. The authors concluded that routine triple-phase imaging of breast cancer patients in whom liver metastases were suspected was not warranted, except at the time of initial examination when triple-phase technique is useful for characterizing focal lesions (e.g., cysts, hemangiomas, focal nodular hyperplasia) that may be encountered.
Magnetic Resonance Imaging
The PV phase occurs 45 to 75 seconds after the start of bolus contrast injection, and during this phase, the liver parenchyma is maximally enhanced, as well as the portal and hepatic veins. This phase is optimal for depiction of most hypovascular liver lesions, including most metastases.
FINDINGS OF LIVER METASTASES
Ultrasound
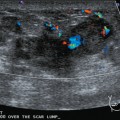
Liver metastases on ultrasound may manifest as solitary or multiple lesions, which most commonly are hypoechoic compared with the normal liver (Figure 1), or as diffuse inhomogeneity of the liver parenchyma (Figure 2).
Computed Tomography
On CT, breast cancer liver metastases typically are hypodense relative to normal liver on noncontrast series, may display rim enhancement or be hypervascular compared with the poorly enhanced liver on the hepatic arterial phase of enhancement, and on the portal venous phase, are hypodense relative to the enhancing liver (Figure 3).

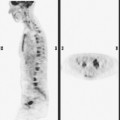
FIGURE 3 CT images of liver metastases from an 83-year-old woman (same patient as in Figure 2) who developed a malignant pleural effusion and liver metastases 20 years after undergoing mastectomy for breast cancer. At the time of this study, the patient had known metastatic breast cancer for 3 years and had been relatively asymptomatic, first on letrozole (Femara) and then on fulvestrant (Faslodex), but progressive disease was suspected based on rising tumor markers. A, Unenhanced abdominal CT scan (acquired with arms at sides during tidal respiration for attenuation correction of PET) shows liver metastases as hypodense lesions compared with rest of the liver. B, Arterial phase enhanced CT scan shows three similar-appearing metastases, with thick peripheral enhancement and central hypodensity. The large hepatic veins are not enhanced during the HA phase of enhancement. C, PV phase enhanced CT image, same level, shows the lesions as larger hypodense regions, and an additional hypovascular metastasis is now seen in the peripheral right lobe, anterior segment, which was not previously seen on unenhanced or HA phase enhanced CT scans.
Magnetic Resonance Imaging
On T1-weighted sequences, the liver normally is brighter than the spleen. Most liver lesions that are not of hepatocellular origin (including the most commonly encountered liver lesions, namely cysts, hemangiomas, and metastases), are hypointense compared with the liver parenchyma on T1-weighted sequences. Lesions that are of hepatocellular origin (such as hepatocellular carcinoma, adenomas, and focal nodular hyperplasia) are often isointense or hyperintense to liver parenchyma on T1-weighted sequences. A variety of T1-weighted sequences are available, including conventional spin echo, turbo or fast spin echo (depending on the manufacturer), and gradient echo. Gradient echo T1-weighted sequences may be acquired with the echo time (TE) varied so that fat and water protons are in or out of phase. Such acquisitions are sensitive to the presence of microscopic fat and are very useful for identifying fatty infiltration, focal areas of fat deposition or sparing, and nodular forms of steatosis (Figures 4 and 5). On in-phase imaging, water and fat signal in the same voxel are additive, whereas on out-of-phase images, water and fat signal in the same voxel are opposed, leading to a loss of signal, which identifies fat-containing regions. The same principle is applied in the use of in-phase and out-of-phase imaging to confirm the identity of lipid-rich adrenal adenomas.


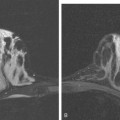
FIGURE 5 Abdominal MRI was then obtained to further assess the indeterminate liver lesions seen on CT in Figure 4. A, Axial half-Fourier acquisition single shot turbo spin echo (HASTE) (T2-weighted) shows the lesion to be brighter than the liver, similar to the spleen (arrow). B, Axial STIR does not clearly show the lesion. C, In-phase, T1-weighted axial image shows the lesion to be slightly hyperintense compared with the rest of the liver. D, Out-of-phase, T1-weighted axial image shows the pivotal observation: there is a marked loss of signal of the lesion, indicating fat content. The other lesions behaved in a similar fashion. E, Axial fat-saturated, T1-weighted, gradient echo image before contrast administration shows the lesion as hypointense. F, PV phase, enhanced, fat-saturated, T1-weighted, gradient echo image shows the lesion as hypointense compared with the liver. No vascular displacement is evident. G, Coronal HASTE (T2-weighted) image shows two of the four similar-appearing lesions in the left lobe. H, Coronal fat-saturated, T1-weighted, gradient echo image of the same two lesions. Hyperechoic ultrasound lesions most commonly represent hemangiomas, but focal fat is also in the sonographic differential diagnosis. These lesions show none of the characteristic imaging features of hemangiomas on CT or MRI. The critical observation to make is the loss of signal of the lesions between in-phase and out-of-phase, T1-weighted, gradient echo imaging, confirming mass-like accumulations of focal fat. This was biopsy-confirmed macrovesicular steatosis.
Fluid-sensitive sequences include T2-weighted and STIR sequences, which can be performed with or without fat saturation. Most liver lesions are hyperintense compared with liver parenchyma. The degree of hyperintensity is helpful in characterization of lesions, with cysts and hemangiomas more hyperintense and “fluid” in signal intensity than metastases and other more “solid” neoplasms. A good rule of thumb: the signal intensity of cysts and hemangiomas should be analogous to the cerebrospinal fluid (CSF) signal, whereas that of metastases and other neoplasms will approximate the signal intensity of the normal spleen.
Dynamic, enhanced imaging consists of repetitive performance of breath-hold, three-dimensional, fat-saturated, T1-weighted, gradient echo volumetric sequences, before, at 25 seconds, at 60 to 70 seconds, and at 2 or more minutes after bolus administration of gadolinium chelate contrast media. The presence or absence of contrast enhancement and the pattern and timing of enhancement generally enable reliable differentiation of benign cysts and hemangiomas from metastases.3
As previously noted, breast cancer metastases have been variably considered in the literature to be hypervascular metastases. Danet and associates looked at a series of liver MRIs obtained on 165 consecutive patients with untreated liver metastases, from a variety of primary tumors, including 16 patients with breast cancer.4 In this series, 69% of the breast cancer patients (11 of 16 patients) had metastases that were hypervascular, defined as enhancement greater than the liver on HA phase of enhancement, comparable to the pancreas. Smaller lesions (<1.5 cm) showed homogeneous hypervascular enhancement, whereas larger lesions (>3 cm) tended to show a peripheral ring of enhancement during the HA phase. In this series, 31% of the breast cancer patients (5 of 16) showed a hypovascular pattern of enhancement of liver metastases. Even for hypovascular metastases, the most common enhancement pattern seen during arterial dominant imaging was a faint peripheral ring of enhancement. The peripheral ring of enhancement was the most common enhancement pattern seen for all untreated metastases during arterial phase imaging in this series, and was seen in both hypervascular and hypovascular metastases and in 72% of the patients overall. This is considered a specific enhancement pattern for metastases. Incomplete central progression of enhancement was the most common pattern seen on delayed imaging in both hypervascular and hypovascular metastases, and was seen in 63% of the patients in this series of untreated metastases from a variety of primaries.
FREQUENCY OF LIVER INCIDENTALOMAS
On imaging of the liver for suspected metastases, a variety of benign “incidentalomas” will be encountered. These must be accurately characterized, reported, then dismissed. It is useful to gain perspective on the scope of this issue by reviewing series from the literature that address the incidence and significance of small focal liver incidentalomas. Depending on the series, small focal liver lesions have been variably defined as less than 10 or 15 mm. Jones and colleagues demonstrated that small focal liver lesions (<15 mm in this series) are common, found in 17% of 1454 consecutive outpatients undergoing contrast-enhanced abdominal CT over a 1-year period.5 Eighty-two percent of the patients with small focal liver lesions had a malignancy history. Of these, lesions were found to be benign in 51%. This report found that in patients with no cancer history, small focal liver lesions are almost invariably benign.
Even in patients with a malignancy history, small focal liver lesions (<10 mm in the series of 2978 cancer patients undergoing CT over a 2-year period by Schwartz and associates) were benign 80% of the time.6 In this series, focal liver lesions proved to be metastases in about 12% of patients overall and in 22% of patients when the malignancy history was breast cancer. However, this series did not include a control group of breast cancer patients without such lesions.
Krakora and colleagues evaluated the prognostic significance of the presence of small (<15 mm), hypoattenuating liver lesions on CT in breast cancer patients.7 This was a retrospective review of 153 breast cancer patients who underwent serial abdominal CT, who did not have definite liver metastases at initial CT. Of these patients, 35% (54 of 153) had one or more small hypoattenuating liver lesions on initial CT. Twenty-eight percent (43 of 153) developed definite liver metastases on subsequent CT. These included 28% of patients (15 of 54) with hypoattenuating liver lesions on initial CT and 28% (28 of 99) without such lesions. No association was demonstrated between the presence, size, or number of small hypodense liver lesions on initial CT and the subsequent development of liver metastases. The authors concluded that in breast cancer patients, the presence of small, hypoattenuating liver lesions on CT, without other evidence of hepatic metastases, was not associated with an increased risk for subsequently developing liver metastases.
Khalil and associates looked at the prevalence and significance of hepatic lesions considered to be too small to characterize (TSTC), which were identified on contrast-enhanced CT obtained in breast cancer patients.8 At least one hepatic lesion considered TSTC (and no definite metastasis) was identified in 29% (277 of 941) of their patients. Subsequent imaging in 69% of patients (191 of 277) showed no change in 92% (175 of 191) or lesion nonvisualization in 4% (8 of 191). Interval enlargement of a lesion previously considered TSTC was noted in 6 of 191 patients (3%). Of these 6, 3 were metastatic breast cancer, 1 was metastatic pancreatic cancer, 1 was a growing cyst, and the final lesion was indeterminate. In this series, liver lesions that were initially TSTC were benign 93% to 97% of the time.
Similar series have looked at the incidence of benign liver incidentalomas detected on liver imaging by MRI in patients with breast cancer. An interesting series was reported by Noone and associates, who looked at 34 patients referred for liver MRI evaluation for suspected metastases at the time of initial breast cancer diagnosis.9 The patients were referred for characterization of liver lesions noted on CT or ultrasound, or for liver function test abnormalities. A full third of the patients had benign lesions identified, including 11 (32%) with benign lesions only and an additional 2 patients with benign lesions coexisting with malignant lesions. The benign lesions were hemangiomas (7), cysts (5), adenomas (2), and one case each of focal fat deposition and focal fatty sparing. Three of 34 patients (9%) had multiple types of benign liver lesions.
Even when not directly evaluating the liver, it may be partially visualized during breast MRI (depending on the coil coverage). Accordingly, even the full-time breast imager needs to be familiar with focal liver lesions (Figure 6). In this setting, the imaging information will not be as complete as with a dedicated abdominal examination. It is important to have an understanding of when there is sufficient information to characterize a liver lesion or not. Similarly, not infrequently, focal liver lesions may have to be assessed based on a single phase of CT enhancement (usually portal venous), such as in metastases screening CT in patients with new diagnoses of locally advanced breast cancer (Figure 7).