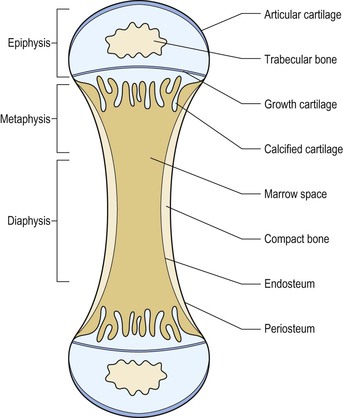
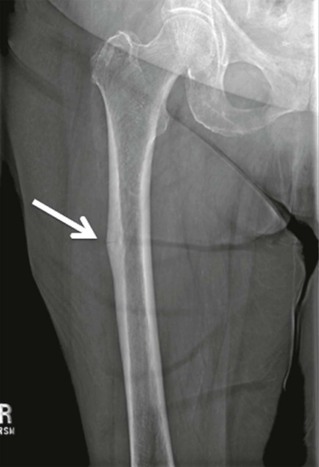
Thomas M. Link, Judith E. Adams Though bone appears rigid and inert, it is a highly metabolically active tissue, which is constantly remodelled with osteoblasts building bone and osteoclasts resorbing bone. This dynamic process allows the bone to be an extremely strong tissue that withstands the load-bearing requirements of the skeleton. Bone consists of crystals of hydroxyapatite embedded within an organic matrix, principally consisting of triple helical fibres of Type I collagen. Bones are generally divided into flat and tubular bones. Tubular bones are designed for weight bearing. Flat bones protect internal organs. Anatomically, bone is found in two forms: Bones remodel from birth to maturity, maintaining their basic shape, repairing following fracture and responding to mechanical stresses throughout life. The strength of bone is related not only to its hardness and other physical properties but also to the architectural arrangement of the compact and trabecular bone. The skeleton contains 99% of the total body calcium and therefore plays a vital role in the maintenance of calcium homeostasis. Osteoblasts are bone-forming cells, which synthesise and secrete Type I collagen and mucopolysaccharides to form layers of bone matrix (osteoid) which subsequently becomes mineralised. Osteoblasts also synthesise collagenase, prostaglandin E2 (PGE2), and bone-associated proteins, osteocalcin and osteonectin. Osteoblasts have receptors for parathyroid hormone, vitamin D, prostaglandin E2 and glucocorticosteroids. Osteocytes are derived from the osteoblast and are initially present on the surface of bone but subsequently become encased within bone. Each osteocyte lies within a lacuna and is interconnected to other osteocytes and osteoblasts by cytoplasmic extensions within canaliculi. The osteocyte has a role in maintenance of bone matrix, which is facilitated by the transport of material and fluid via these canaliculi. Osteocytes respond to biomechanical loading, calcitonin and parathyroid hormone, and so play an important role in maintaining constant levels of calcium within the body fluids. Osteoclasts are multinuclear giant cells that resorb both calcified bone and cartilage and derive from the mononuclear phagocytic cell line of haematopoietic stem cells. Osteoclasts lie on the surface of bone, causing active resorption and forming Howship’s lacunae. Osteoclasts in contact with bone develop motile microvilli, which cause the cell to adhere to the bone surface and result in a microenvironment between the osteoclast and the mineralised bone. This brush border of microvilli increases with activation by such factors as prostaglandin, vitamin D and parathyroid hormone. Osteoclasts secrete acid hydrolases and neutral proteases, which degrade the bone matrix following its demineralisation. Figure 50-1 shows the different bone cells and their function in remodelling the bone. Bone formation (osteoblastic activity) and bone resorption (osteoclastic activity) constitutes bone turnover, a process, which takes place on bone surfaces and continues throughout life (Fig. 50-1). Bone formation and resorption are linked in a consistent sequence under normal circumstances. Precursor bone cells are activated at a particular skeletal site to form osteoclasts, which erode a fairly constant amount of bone. After a period of time the resorption stops and osteoblasts are recruited to fill the eroded space with new bone. This coupling of osteoblastic and osteoclastic activity constitutes the basal multicellular unit (BMU) of bone. In healthy young adults the resorptive phase of the turnover cycle lasts about 30 days and the formation phase about 90 days. The length of the turnover cycle increases in later life and the rate of bone turnover is reduced. Uncoupling of the process (excessive osteoclastic resorption or defective osteoblastic function) results in a net loss of bone (osteoporosis). If there is both increased bone resorption and formation, this constitutes increased bone turnover. Woven immature, instead of mature lamellar, bone is laid down, as in Paget’s disease of bone. Increased activation frequency of resorption units also results in high turnover state (hyperparathyroidism, postmenopausal bone loss). Bisphosphonate therapy reduces the activation of resorption units by inhibiting osteoclasts, and the reversal in the mineral deficit contributes to increase in bone mineral density (BMD). Defective osteoclastic function prevents normal bone resorption, which is essential to maintain bone health by continuous slow renewal throughout life. Defective osteoclastic function in some diseases (i.e. osteopetrosis) can result in abnormal bone modelling and sclerosis on radiographs; these bone are more brittle and susceptible to fracture. Bone resorption by osteoclasts is a single-stage process in which collagen and mineral are removed together. Bone formation is a two-stage process: osteoblasts lay down osteoid, which subsequently becomes mineralised. Prerequisites for normal mineralisation are vitamin D (1,25[OH]2D3), normal levels of phosphorus and alkaline phosphatase, adequate intake of calcium and a normal pH. Defects in the mineralisation process will result in rickets or osteomalacia. Early in fetal development the framework of the skeleton is in place but without mineralisation. At about 26 weeks of gestation the long bones assume their future shape and proportion. Bones grow in size and change in shape during childhood and adolescence, particularly during the pubertal growth spurt. Skeletal growth occurs primarily by endochondral ossification at the metaphyses and epiphyses (Fig. 50-2). The primary centre of ossification in the tubular bones is in the centre of the cartilaginous template. The secondary, later developing, centres (epiphyses) are located at the ends of the developing bones. In endochondral ossification there is hypertrophy of cartilage cells and glycogen accumulation. Subsequently, these cells undergo degeneration and become calcified (the provisional zone of calcification). The deeper perichondrial cells transform into osteoblasts through a process of intramembranous ossification. These osteoblasts, and vascular tissue, invade the cartilaginous matrix and lay down osteoid, which becomes mineralised. Osteoblasts become trapped within the developing bone and transform into osteocytes. Increase in bone length takes place at the metaphyses of long bones, which adjoin the cartilaginous growth plate. This cartilaginous growth plate remains between the ossification primary and secondary centres until growth ceases and skeletal maturity is reached. The remnant of the cartilage of the epiphyses adjacent to the articular surface becomes the articular cartilage of the adjacent joint. If endochondral ossification ceases for some reason (illness, nutritional deprivation) then the zone of provisional calcification present at that particular stage of skeletal development may remain as a thin white line (Harris growth arrest line) on radiographs (Fig. 50-3). Bones consist of an outer shell of compact (cortical) bone with a central cavity which contains the marrow space, and ‘lace-like’ trabecular bone which is prominent in the axial skeleton and at the ends of long bones. Compact cortical bone constitutes about 80% of skeletal mass. Cancellous (trabecular) bone constitutes 20% of total skeletal mass, but contributes importantly to skeletal strength. The bone trabeculae are arranged to resist tensile deforming stresses, either from weight bearing or muscular activity. The number, thickness and distribution of trabeculae are related to biomechanical loading. Trabeculae provide a large surface area on which metabolic processes can take place and have a higher rate of turnover and richer blood supply than compact bone. Around the cortex of the bone is a layer of periosteum and adjacent to the marrow cavity is its endosteal surface. Excessive osteoclastic activity (e.g. in hyperparathyroidism) causes resorption of cortical bone, which may be visible radiologically (cortical ‘tunnelling’ and erosions) and is indicative of increased bone turnover. Resorption and formation takes place not only within the cortex of bone but also at the periosteal and endosteal surfaces. As we age, more bone is removed at the endosteal surface than is replaced, resulting in a net loss of bone at this site. This causes the marrow cavity to enlarge and the bony trabeculae to become thinner—some may ultimately disappear. At the periosteal surface, resorption takes place, which is important in maintaining the normal shape of bones as they grow in length. There is a net gain of bone at the periosteal surface throughout life, so that tubular bones progressively increase in width as age advances. The bones grow during the first two decades of life with a pubertal spurt during adolescence (Fig. 50-4). Skeletal maturity is achieved at an earlier age in girls (16–18 years) than in boys (18–20 years). Some disorders (hypothyroidism, chronic ill health) may retard skeletal development. Skeletal maturation is assessed radiologically from a non-dominant hand radiograph which is then compared with an atlas of hand radiographs of normal American Caucasian boys and girls of different ages1 or using the Tanner and Whitehouse bone score (TW2) method, which assesses changes in presence, size and shape of certain bones with age.2 Following attainment of skeletal maturity, bone is consolidated and peak bone mass is achieved. For cortical bone this is reached at about 35 years of age, and a little earlier for trabecular bone (Fig. 50-4). Although the long bones grow in length at the metaphyses, they are remodelled in shape during development by endosteal resorption and periosteal apposition. The size and shape of the skeleton and its individual bones are determined by genetic factors, but are influenced by endocrine and local growth factors, nutrition and physical activity. Remodelling allows the skeleton to adjust to mechanical forces to which it is exposed. There is considerable variation in skeletal size and weight, both within and between races. Black races have larger and heavier bones than whites, and Chinese tend to have lower skeletal mass and smaller size. Although genetic factors are important, they are modified by environmental differences such as diet and physical activity. In mature healthy adults, bone turnover for the whole skeleton is about 2% per year, with maintenance of a constant bone mass. Bone formation is increased during periods of rapid growth and stimulated by physical activity, growth hormone and thyroid hormone. Bone formation is decreased as a consequence of immobilisation, undernutrition, deficiencies of thyroid and growth hormone and glucocorticoid excess. After the attainment of peak bone mass, bone loss, particularly of trabecular bone, is believed to occur from the third decade of life. Bone loss is a phenomenon, which occurs in all races (Fig. 50-4). Generally both men and women lose bone as they grow older, but women lose more than men. Women lose approximately 15–30% of their total bone mass between maturity and the seventh decade, whereas men lose only about half this amount. After the age of 35, women lose bone at an annual rate of approximately 0.75–1.0%, which increases to a rate of 2–3% in the postmenopausal period. This loss affects both cortical and trabecular bone, but the effect on trabecular bone predominates. In contrast, cortical bone is well preserved until the fifth decade of life when there is a linear loss in both sexes, such that men lose about 25% of their cortical bone whilst women lose about 30%. Low bone mineral density may be the result of either low peak bone mass attainment or subsequent accelerated bone loss. The most common metabolic disorders of bone are: osteoporosis, in which there is a deficiency of bone mass leading to insufficiency (low-trauma) fractures; rickets and osteomalacia, in which there is a defective mineralisation of bone osteoid due to vitamin D deficiency, hypophosphataemia, lack of alkaline phosphatase or calcium, or severe acidaemia; and hyperparathyroidism, in which a tumour or hyperplasia of the parathyroid glands causes increase in parathyroid hormone production and stimulation of osteoclasts. Other metabolic bone disorders include osteogenesis imperfecta, hyperphosphatasia and osteopetrosis. Paget’s disease is not strictly a metabolic bone disease, since it can be monostotic or polyostotic and does not involve the entire skeleton, but because it involves increased bone turnover it is often included in this group of disease. Osteoporosis is the most common metabolic bone disease, with increasing significance as the population ages. Fragility fractures due to osteoporosis are one of the most significant challenges to public health worldwide. The elderly represent the fastest-growing age group, and the yearly number of fragility fractures will increase substantially with continued ageing of the population.3 Approximately 50% of women and 20% of men older than 50 years will have a fragility (insufficiency) fracture in their remaining lifetime in Caucasian populations.4 Osteoporosis is defined as a skeletal disorder characterised by compromised bone strength predisposing a person to an increased risk of fracture.5 Bone strength primarily reflects the integration of bone mineral density (BMD) and bone quality.5 BMD is expressed as grams of mineral per area or volume, and in any given individual is determined by peak bone mass and amount of bone loss. Bone quality refers to architecture, turnover, damage accumulation (e.g. microfractures), and mineralisation.5 Though BMD is only in part responsible for bone strength, dual energy X-ray absorptiometry (DXA) measurements of BMD have been universally adopted as a standard to define osteoporosis in terms of bone densitometry. In 1994 the World Health Organization (WHO)6 used T-scores to classify and define BMD measurements. A T-score is the standard deviation of the BMD of an individual patient compared to a young, healthy reference population, matched for gender and ethnicity. According to the WHO, normal, osteopenic and osteoporotic BMD are differentiated. Normal: BMD above (≥) –1 standard deviation (sd) of the young adult reference mean (peak bone mass). Osteopenia: BMD between (<) –1 and (>) –2.5 sd below that of the young adult reference mean. Osteoporosis: BMD more than (≤) –2.5 sd below the young adult reference mean. It should be noted that even in the absence of osteoporotic BMD the presence of one or more low-impact fragility fractures is considered as a sign of severe osteoporosis7 and that not infrequently in these patients the BMD measured with DXA may be in the normal or osteopenic range. The osteoporosis/osteopenia definition is also applied to older men, but it is not used in men younger than 50, premenopausal women and children or adolescents who have not yet reached peak bone mass. In these patient groups, low bone mass would appropriately be defined as a BMD which is more than 2 standard deviations below the mean BMD matched for age, gender and ethnicity (http://www.iscd.org/visitors/positions/OP-Index.cfm and).8–10 The WHO definition is not applicable to other bone densitometry techniques (quantitative computed tomography, QCT; quantitative ultrasound, QUS) or other anatomical sites (e.g. calcaneus). Osteoporosis should not be considered as a single disease entity, but rather an end result of many disease processes (Table 50-1). It may result from defective skeletal accretion during bone growth and development. Alternatively, it may result from disease processes in which bone resorption exceeds new bone formation, resulting in a net loss of bone mass and consequent compromise to skeletal strength. TABLE 50-1 Main Causes of Osteoporosis Osteoporosis is radiographically characterised by decreased radiodensity of bone (Fig. 50-5). However, it should be noted that radiographs are very limited in assessing the amount of bone loss11 and only advanced bone loss can be identified. Descriptive terms that have been used to describe reduced bone density in the absence of fragility fractures are ‘osteopenia’ or ‘demineralisation’. The latter is an incorrect term since mineral and collagen are both reduced in osteoporosis. Reduced bone density is often more prominent in areas of the skeleton rich in trabecular bone, particularly in the axial skeleton (vertebrae, pelvis, ribs and sternum). Eventually, changes may also be evident in the bones of the appendicular skeleton. Trabeculae become thin and may disappear completely; they may be sparse, but those that remain may become thickened due to stresses to which the skeleton is exposed (Fig. 50-6). The cortex becomes reduced in width through endosteal bone resorption, and in states of increased bone turnover there will be intracortical tunnelling and porosity. Osteoporotic bone is less able to withstand the stresses to which the skeleton is exposed compared to normal bone, and this leads to the cardinal clinical feature of low trauma fractures. Such fractures can occur at any skeletal site, but they are most common in sites of the skeleton rich in trabecular bone, particularly the vertebrae, the distal forearm and the proximal femur. These fractures may be associated with considerable pain and deformity. In individuals who suffer hip fractures 20% will die within the next year and 20% will require permanent nursing home care.4 Even if age-adjusted incidence rates for hip fractures remain stable, the estimated number of hip fractures worldwide will rise from 1.7 million in 1990 to 6.3 million in 2050.3 It is important to identify patients with osteoporosis and at increased risk of fracture as there are now therapies available (bisphosphonates, selective oestrogen receptor modulators (SERMs), strontium ranelate; teriparatide (parathyroid hormone) and denosumab) which cause relatively small increases in bone mineral density (4–12%) but more importantly reduce future fracture risk by between 40 and 70%.12,13 Related to biomechanical forces through the spine, as trabeculae are lost, the process of osteoporosis particularly involves the horizontally orientated secondary trabeculae. The vertical trabeculae actually become more prominent and thickened.14 This results in a vertical ‘striated’ appearance to the vertebral body on lateral spinal radiographs and cross-sectional imaging studies. Figure 50-6 shows this pattern in sagittal and coronal reformations of CT images, which was also termed hypertrophic atrophy. This feature is generally seen in several, or all, of the vertebrae when it is related to osteoporosis, which serves to distinguish a similar appearance in a single vertebral body when it is related to haemangioma. Vertebral fractures are the most common of osteoporotic fractures (Fig. 50-7). The anterior and central mid portion of the vertebrae withstand compression forces less well than the posterior and outer ring elements of the vertebrae, resulting in wedge or end-plate fractures or, less commonly, crush fractures.15 Vertebral fractures can be graded as mild (20–25% change in shape; grade 1), moderate (26–40% change in shape; grade 2) and severe (>40% change in shape; grade 3)16 (Fig. 50-8). This semi-quantitative grading (SQ) method is the one currently most frequently applied to define the prevalence and incidence of vertebral fractures in epidemiology studies and pharmaceutical trials of the efficacy of new osteoporosis therapies. The more severe the grade of vertebral fracture, the greater the risk of future fracture. Vertebral fractures are powerful predictors of future fracture (hip ×2; vertebral ×5). If vertebral fractures are present it is therefore extremely important that they are accurately and clearly reported by radiologists as fractures; other terms such as ‘deformities’, ‘collapse’, must be avoided. There is evidence that vertebral fractures are being under-reported by radiologists, with the result that patients who should be receiving treatment to reduce their risk of future fractures are not being identified.17–19 As a consequence, a joint initiative was launched in 2002 between the International Osteoporosis Foundation (IOF) and the European Society of Skeletal Radiology (Osteoporosis Group) to improve the sensitivity and accuracy of reporting of vertebral fractures by radiologists. Vertebral fractures may occur as an acute event related to minor trauma and be accompanied by pain, which generally resolves spontaneously over 6–8 weeks. This resolution of symptoms serves to distinguish osteoporotic vertebral fractures from similar events due to more sinister abnormalities, such as metastases, in which the symptoms are more protracted. However, 30% or more of vertebral fractures may be present in asymptomatic patients. Osteoporotic fractures occur most commonly in the thoracic and thoracolumbar regions and result in progressive loss of height in affected individuals. Osteoporotic fractures are uncommon above T7; if fractures are present above this anatomical region, metastases should be considered. Also if the posterior aspect of the vertebral body is fractured metastases or myeloma should be the first differential diagnosis. Wedging of multiple vertebral bodies in the thoracic spine can lead to increased kyphosis (dowager hump) which, if severe, may result in the ribs abutting on the iliac crests with compromise of respiratory function and reduced quality of life. Vertebroplasty and kyphoplasty have selected application in patients with osteoporotic vertebral fractures that are persistently painful.20 The technique is performed mostly by radiologists and orthopaedic surgeons. Vertebroplasty is the injection of cement (methylmethacrylate) into a fractured vertebral body as a means of treating pain. Injection is generally made by passing the introduction needle through the pedicles. Kyphoplasty is the injection of cement into the fractured vertebral body after a balloon has been used to form a cavity and to decompress the fracture and correct some of the deformity21 (Fig. 50-9). Both techniques are intended to relieve pain in patients who have not responded to conservative measures. It has been suggested that vertebroplasty/kyphoplasty should be performed in the first 4–6 weeks after presentation with pain, which requires opiates for pain relief and/or hospital admission.22 Certainly patients with proven osteoporosis should always be commenced on bone protective/bone enhancing therapy when vertebroplasty is to be performed. Patient selection is crucial to the outcome of the procedure. The pain should arise from vertebral fractures that are temporally related to the onset of symptoms. Magnetic resonance imaging (MRI) with fluid-sensitive sequences and fat suppression can aid the identification of more acute fractures showing bone marrow oedema, typically along the end-plates, in acute and subacute fractures with ongoing bony remodelling. Two recent trials comparing vertebroplasty with a sham procedure suggested that improvements in pain and pain-related disability associated with osteoporotic compression fractures in patients treated with vertebroplasty were similar to the improvements in a control group.23,24 Conversely a study published in 2010 concluded that pain relief after vertebroplasty was immediate, sustained for at least a year, and was significantly greater than that achieved with conservative treatment, at an acceptable cost.25 The chance of successful pain relief by vertebroplasty for osteoporotic fracture is between 70 and 95%.26 In a previous study kyphoplasty and vertebroplasty demonstrated similar good clinical outcomes during the 12-month follow-up; but kyphoplasty offered a higher degree of spinal deformity correction and resulted in less cement leakage than vertebroplasty.27 There are potential risks of vertebroplasty; these may be needle related (pedicle fracture, needle breakage, pneumothorax, haemorrhage, infection), cement related (root compression, cord compression, pulmonary cement emboli), procedure related (pulmonary fat emboli, rib or vertebral fracture), sedation related (respiratory arrest, airway injuries, cardiac arrest) or drug related (allergy). The overall complication rate from reports suggests that symptom-inducing or potentially serious complications occur in approximately 2% of patients treated for osteoporotic fracture.28 Most cases are treated under conscious sedation with analgesia, but in some patients general anaesthesia is required. For needle placement, high-quality fluoroscopy in either a biplane or C-arm configuration is recommended. Needles, injector sets and cements are now manufactured specifically for vertebroplasty. Care should be taken to ensure a sterile environment. All patients treated by vertebroplasty or kyphoplasty for osteoporotic compression fractures should be under the care of a clinician with special interest in osteoporosis management and on appropriate medical therapy to reduce future fracture risk.13 Fractures that occur in osteoporosis generally heal well with satisfactory callus formation. In some sites the presence of multiple micro-fractures and callus formation can cause osteosclerosis on a radiograph; this must be distinguished from other pathologies such as bone metastases. These insufficiency fractures occur in particular anatomical sites, including the symphysis pubis, the sacrum, pubic rami and calcaneus. Other sites involved are the sternum, supra-acetabular area and elsewhere in the pelvis, femoral neck, humerus and proximal and distal tibia. Some of these fractures may be accompanied by considerable osteolysis, particularly those involving the symphysis pubis. Fragility fractures of the pelvis have been misinterpreted as neoplastic lesions and several previous studies29–31 focused on the importance of correctly diagnosing sacral fractures to avoid misguiding patient management, which may produce dangerous and costly interventional procedures. Cross-sectional imaging techniques (CT and MR imaging) may help to differentiate insufficiency fractures from other pathologies (Fig. 50-10). On radionuclide bone scintigraphy there is increased uptake in regions of acute insufficiency fractures. When the sacrum is involved, there is often a characteristic H pattern (Honda sign) of radionuclide uptake. CT and MRI are particularly helpful in defining the fracture lines of insufficiency fractures involving the sacrum (fractures usually occur parallel to the sacroiliac joint) and in the calcaneus. In these sites fractures may not be identified on radiographs because of complex anatomy and overlying structures. MRI is helpful in differentiating vertebral fractures resulting from osteoporosis from those caused by other pathologies (myeloma, metastases). It should be noted that MR is more sensitive than CT in diagnosing pelvic insufficiency fractures.30 During the past decade a number of publications focused on osteoporotic insufficiency fractures and demonstrated that findings previously defined as osteonecrosis are actually insufficiency fractures.29,30,32–35 Previously, the term’ spontaneous osteonecrosis of the knee (SONK), was used to describe an osteochondral lesion which was typically observed at the medial femoral condyle but which is now considered to be an insufficiency fracture. Both insufficiency fractures at the medial femoral condyle of the knee and femoral head are frequent findings in older individuals and indicate increased fragility of the skeleton (Fig. 50-11). Recently atypical subtrochanteric and femoral shaft fractures have been reported in older individuals and on long-term bisphosphonate therapy.36–38 Coexisting factors have been discussed in the aetiology of these fractures such as co-morbidities (e.g. vitamin D deficiency, chronic obstructive airways disease, rheumatoid arthritis, diabetes) and other drugs (e.g. glucocorticoids, proton pump inhibitors).36–38 The task force of the American Society for Bone and Mineral Research38 identified a number of major and minor clinical and imaging features of these fractures which include location in the subtrochanteric region and femoral shaft, transverse or short oblique orientation, minimal or no associated trauma, a medial spike when the fracture is complete, absence of comminution, focal cortical thickening and a periosteal reaction of the lateral cortex (Fig. 50-12). There may be prodromal symptoms such as dull or aching pain in the groin or thigh. As these stress fractures may be bilateral in up to 50% of patients and occur anywhere in the femoral shaft, if suspected, imaging should include both femora in their entirety. These fractures are increasingly diagnosed, can occur in bisphosphonate naïve patients and not infrequently only lateral cortical thickening is evident. This may progress to a complete fracture and is therefore a critical finding, which needs to be communicated to the clinician. These incomplete stress fractures are treated with prophylactic internal fixation surgery. The relation of this complication with long-term bisphosphonate therapy has led to consideration of a drug ‘holiday’ at 5-year review. If the BMD T-score is above –2.5, treatment may be discontinued for two years and then the necessity for recommencement is considered; however, there are no scientific data on which to base such recommendations. Osteoporosis can be classified as generalised, regional (involving a segment of the skeleton) or localised. This can occur in disuse and reflex sympathetic dystrophy (RSD/regional pain syndrome) following fracture, or related to other pathologies (primary and secondary tumours). Chronic disuse is characterised by a uniform pattern of bone loss; acute immobilisation causes more focal and irregular bone formation and resorption. This results in different patterns of bone loss, which include diffuse osteopenia, linear translucent bands, juxta-articular speckled radiolucent areas and cortical bone resorption.39 MRI findings in disuse osteopenia are also typical and include accentuation of vertical trabecular lines, presence of subchondral lobules of fat, horizontal trabecular lines, prominence of blood vessels, and dotted and patchy areas of high signal intensity on fluid-sensitive sequences40 (Fig. 50-13). RSD/complex regional pain syndrome is a clinical syndrome that can occur in children and adults and may be precipitated by a variety of processes. There is overactivity of the sympathetic nervous system, initially causing pain, soft-tissue swelling and hyperaemia, with excessive bone resorption (probably stimulated by cytokines), which occurs particularly in a peri-articular distribution and may simulate malignant disease. The diagnosis of RSD/complex regional pain syndrome relies on clinical evaluation and radiographs. MRI may provide a differential diagnosis between RSD and other bone pathologies as it demonstrates diffuse signal abnormalities with soft-tissue and bone marrow oedema pattern.41 There are also conditions which cause focal or migratory and transient osteoporosis, usually in the region of large joints (hip, knee), the aetiology of which is uncertain. Transient osteoporosis of the hip occurs in younger and middle-aged individuals and middle age, more frequently in men than women. Also, it is typically found in women in the third trimester of pregnancy. There is sudden onset of pain without preceding trauma. Radiographically there is reduction in density of the proximal femur. There may be an underlying abnormality of perfusion of the marrow, which is oedematous. MRI is sensitive in demonstrating bone marrow oedema pattern without focal abnormalities before any radiographic abnormality is evident (Fig. 50-14).42,43 There are many causes of osteoporosis, falling into four categories: 1. Factors which reduce peak adult bone mass 3. Bone loss associated with menopause or hypogonadal state 4. Bone loss that is secondary to other medical conditions and drugs (Table 50-1). This self-limiting form of osteoporosis occurs in prepubertal children and must be differentiated from osteogenesis imperfecta and other forms of juvenile osteoporosis. IJO is a rare disorder, first described by Dent and Friedman,44 and occurs in children aged between 8 and 14 years who have previously been healthy. The disease runs an acute course over a period of 2–4 years, during which there is growth arrest and fractures. There is a wide spectrum of severity, and both cortical and trabecular bone are affected. In the mild form, only one or two vertebral fractures may be present, but in more severe cases fractures involve all the vertebrae and the extremities, particularly the metaphyseal region of the distal tibia. A few affected patients may develop severe kyphoscoliosis, deformities of the extremities, and even die from respiratory failure due to thoracic deformity. The disease is reversible and remits spontaneously. Affected patients may be left with only a mild or moderate kyphosis, short stature and some bone deformity following fractures.44 Investigations indicate uncoupling of the two components of bone turnover due to both increase in resorption and decrease in formation. The important differential diagnosis in children with vertebral fracture is hypercortisolism and leukaemia. Affected patients do not have the blue sclerae characteristic of osteogenesis imperfecta. This heterogeneous condition occurs equally in young men and women.45 The disease generally runs a mild course with multiple vertebral fractures occurring over a decade or more, with associated loss in height. Fractures of metatarsals and ribs are also common, and hip fractures may occur. The cause of the condition is uncertain and in some patients it may simply be that inadequate bone mass has been accrued during skeletal growth. Some affected individuals may have a mild variant of osteogenesis imperfecta. Rarely, osteoporosis may present during pregnancy, but whether this is a causal or coincidental association is unknown. At the time of the menopause, lack of oestrogen will result in some women losing trabecular bone at a rate three times greater than is usual (2–10% per annum). The condition, previously referred to as type I osteoporosis, characteristically becomes clinically evident in women 15–20 years after the menopause. Fractures occur in sites of the skeleton rich in trabecular bone, including the vertebral bodies and distal forearm (Colles’ fracture). This condition, previously referred to as type II involutional osteoporosis, occurs in both men and women of 75 years or older and is due to age-related bone loss. This occurs as a consequence of age-related impaired bone formation associated with secondary hyperparathyroidism caused by reduced intestinal calcium absorption due to decreased levels of 1,25(OH)2D production in the elderly.46 There is reduction in both cortical and trabecular bone. The syndrome manifests mainly as hip fractures and wedge fractures of the vertebrae, but fractures may also occur in the proximal tibia, proximal humerus and pelvis. A large number of conditions may lead to osteoporosis (Table 50-1). Radiologically these may be indistinguishable from age-related osteoporosis. However, some may have specific and diagnostic radiological features (i.e. subperiosteal erosions of the phalanges in primary hyperparathyroidism). In glucocorticoid excess (endogenous and exogenous), there is reduced bone formation due to a direct effect on the osteoblast, and increased osteoclastic activity, probably mediated through secondary hyperparathyroidism, stimulated by reduced gastrointestinal absorption of calcium. There is also evidence that glucocorticoids induce premature apoptosis of both osteoblasts and osteoclasts. The effect is primarily on trabecular bone. Fractures occur particularly in the vertebral bodies and ribs; the latter may heal with profuse callus formation. Fractures may appear relatively rapidly after starting oral glucocorticoid medication, in particular with high doses and in younger patients (Fig. 50-15). A number of inherited disorders of connective tissue may result in osteoporosis. Osteogenesis imperfecta (OI), or brittle bone disease, results from mutations affecting either the COL1A1 or COL1A2 gene of Type I collagen.47 Although the disease is usually apparent at birth or in childhood, more mild forms of the disease may not become apparent until adulthood, when affected individuals may present with insufficiency fractures and osteopenia (Fig. 50-16). Radiographic features vary according to the type of disease and its severity and include osteopenia and fractures, which may heal with florid callus formation, mimicking osteosarcoma. Bones are thin and over-tubulated (gracile), normal in length or shortened, thickened and deformed by multiple fractures. Intra-sutural (Wormian) bones can be identified on skull radiographs. In severe forms of osteogenesis imperfecta the diagnosis may be made before birth by detailed ultrasound in the second trimester. Diagnostic features include cranial enlargement, reduced echogenicity of bone and deformity and shortening of limb bones as a result of intrauterine fractures. Osteogenesis imperfecta is classified based on distinct characteristics, including blue sclerae, the severity of the disorder and the mode of inheritance (dominant, recessive, sporadic/new mutation).48 However, accurate classification is difficult because of phenotypic overlap.47 Affected subjects who do not have dental involvement are designated as group A. Subjects with dentinogenesis imperfecta are designated as group B. This is the mildest and most prevalent form of the disease and may only become apparent in adulthood. There is a history of fractures, generally dating back to childhood. In children the fractures may become radiographically and clinically apparent as the child becomes more active (5+ years), and may take the form of overt fractures or micro-fractures involving the metaphyses. In infancy these features may resemble those found in non-accidental injury.49 The differential diagnosis can usually be resolved by the presence of associated extraskeletal manifestations of osteogenesis imperfecta (blue sclerae, dentinogenesis imperfecta), or evidence of a family history of the condition. Bone biopsy for diagnosis is rarely required. Affected patients are short in stature, only 10% being of normal height, with joint laxity, blue sclerae and presenile hearing loss. Transmission is by autosomal dominant trait. Radiologically the bones are usually reduced in radiodensity, although some patients may have normal bone density. Bones may be gracile, or modelled normally. Vertebral fractures often occur in the fourth decade and when scoliosis is present, it is mild. Affected infants are small for dates with deep blue sclerae and shortened and deformed limbs due to multiple fractures. Fractures involve the ribs and death is usually the result of pulmonary insufficiency. Survival is rare beyond the first three months of postnatal life. Other complications include brain and spinal cord injury. Radiologically, multiple fractures are present with a characteristic ‘concertina’ deformity of the lower limbs. The ribs may appear ‘beaded’ due to multiple rib fractures, which can occur in utero. The cranial vault is severely under-mineralised and may be distorted by moulding, with Wormian (intrasutural) bones in the occipital and parietal region. Platyspondyly is present. Histology reveals defective endochondral ossification at the metaphyses and epiphyses, which appear disorganised with persistent islands of calcified cartilage and under-mineralised bone. There is defective transformation of woven bone to lamellar bone in both the cortical and trabecular skeletal components. Membranous ossification is also deficient, accounting for the marked calvarial thinning. This is inherited as an autosomal recessive trait. Fractures are usually present at birth and involve the long bones, clavicles, ribs and cranium, leading to deformity. Although size at birth is normal, growth retardation is evident in the first year of life and many affected patients only reach 0.9–1.2 m (3–4 ft) in height. As growth proceeds, increasing deformity of the calvarium occurs, with associated facial distortion, dental malocclusion and mild prognathism, basilar invagination and progressive hearing loss. Sclerae are blue at birth but this diminishes with age, and sclerae are white in adults. Vertebral fractures occur at an early age and contribute to the progressive and severe kyphoscoliosis, which develops during childhood. Affected patients tend to be wheelchair bound because of the progressive deformities resulting from fractures. Complications include progressive pulmonary insufficiency due to thoracic distortion. Radiologically, the bones may be slender or broad due to recurrent fractures. Epiphyses are abnormal, with expansion and islands of calcified (‘popcorn’) cartilage. As with other forms of osteogenesis imperfecta, the incidence of fractures declines following puberty. This is inherited as an autosomal dominant trait, can vary in severity and is sometimes confused with either type I or type III OI. There is generally more severe osteopenia and more extensive bone deformity than in type I. The sclerae are blue in children and while this may persist into adulthood, they may also fade to white. Individuals are short in stature with abnormal moulding of the calvarium and basilar invagination in a high proportion of patients. Bones in the axial and appendicular skeleton are osteoporotic and dysplastic, resulting in scoliosis and deformity, particularly of the pelvis. Joint laxity can result in dislocation, particularly of the ankle or knee.
Metabolic and Endocrine Skeletal Disease
Bone Physiology and Pathophysiology
Bone Cells
Bone Formation and Turnover
Bone Growth and Development
Osteoporosis
Definition and Epidemiology
Primary
Secondary

Radiological Features
Spine in Osteoporosis

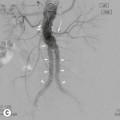
Vertebroplasty and Kyphoplasty
Osteoporotic Fractures
Aetiology
Regional Osteoporosis
Generalised Osteoporosis
Idiopathic Juvenile Osteoporosis (IJO).
Osteoporosis of Young Adults.
Postmenopausal Osteoporosis.
Senile Osteoporosis.
Secondary Osteoporosis.
Osteogenesis Imperfecta
Type I
Type II (Lethal Perinatal)
Type III (Severe Progressive)
Type IV (Moderately Severe)
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Metabolic and Endocrine Skeletal Disease
Chapter 50
FIGURE 50-8 The semiquantitative method of grading of Genant et al,16 which is widely used in epidemiology and pharmaceutical studies. Vertebral fractures are strong predictors of future fractures (×5 for vertebral fracture; ×2 for hip fracture). The higher the grade of vertebral fracture, the higher the risk of future fracture.