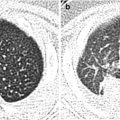
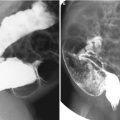
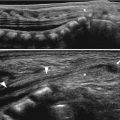
Fig. 11.1
Various appearances of a normal thymus in newborn. (a) “Sail” sign. Note triangular extension laterally that looks like a sail. (b) “Wavy thymus” sign. The arrow indicates the undulating margin of the thymus due to gentle compression by the adjacent anterior rib. (c, d) The prominent thymus mimics a mediastinal mass or left upper lobe parenchymal lesion. On precontrast CT, the thymus is seen as a homogeneous soft tissue structure in the anterosuperior mediastinum with extension posteriorly and laterally along the arch of the aorta. (e, f) On this anteroposterior chest radiograph taken in a rotated position, a large thymus mimics upper lobe atelectasis. Ultrasonography was performed in order to confirm the thymus. Transverse ultrasonographic scan demonstrates linear or dot-like echogenicity within the superior mediastinal soft tissue structure, the characteristic internal pattern of the normal thymus
11.6.2 Hyaline Membrane Disease
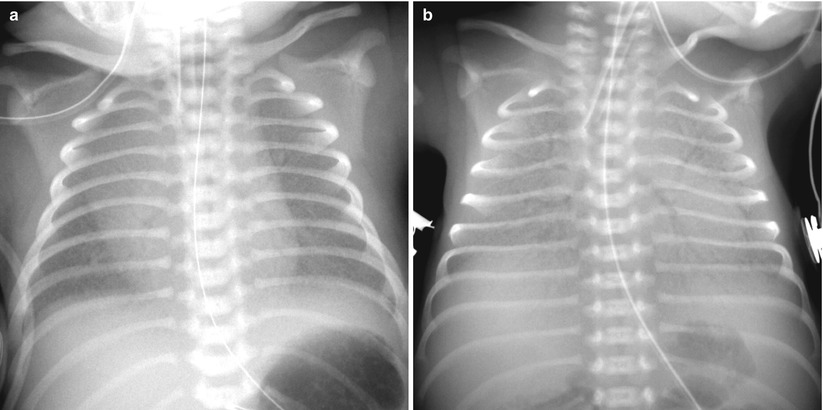
Fig. 11.2
The “classic” appearance of hyaline membrane disease. It may vary in its clinical and imaging severity. (a) Moderate HMD, frontal radiograph shows evenly distributed, fine granular opacification and pulmonary hypoventilation. (b) Severe HMD, frontal radiograph shows white-out with air bronchograms. Note generalized underaeration of the lungs
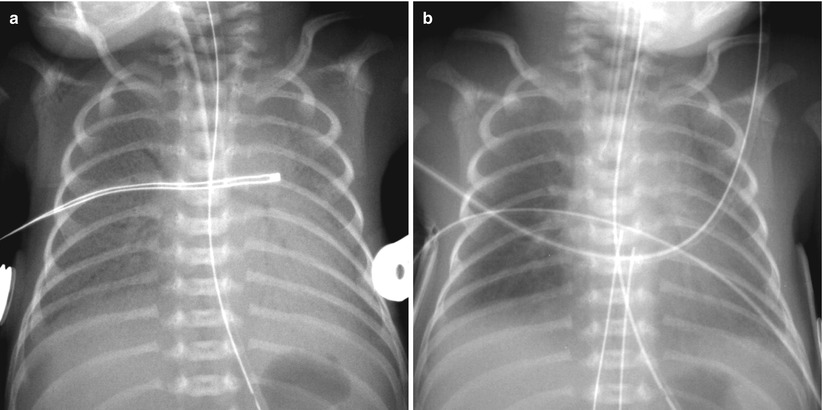
Fig. 11.3
Hyaline membrane disease in a premature neonate of 28 weeks’ gestational age. (a) Anteroposterior chest radiograph obtained on the first postnatal day demonstrates diffuse fine granularities, pulmonary hypoventilation, and air bronchograms. (b) Follow-up infantogram obtained 2 h after surfactant treatment shows clearing of lung opacity, but haziness is still noted in the periphery and the bases of the lungs
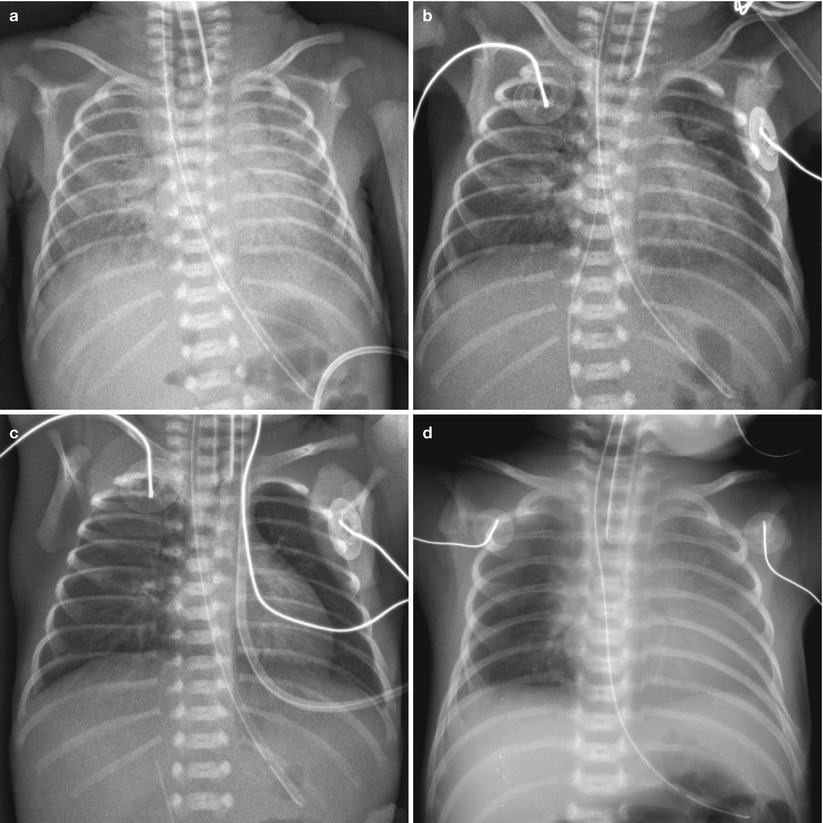
Fig. 11.4
Asymmetric surfactant effect in a newborn with hyaline membrane disease. (a) Initial chest radiograph demonstrates findings of HMD. (b) Frontal chest radiograph obtained after surfactant administration demonstrates multifocal residual parahilar opacities that mimic pneumonia or meconium aspiration syndrome. (c) Repeat chest radiograph after administration of second dose of surfactant shows clearing of opacifications, but asymmetric diffuse faint haziness is noted only in the right lung field. (d) Asymmetric distribution of endotracheal surfactant into the right main stem bronchus in a 1-day-old preterm neonate with HMD. Frontal radiograph obtained 2 h after surfactant administration shows asymmetric clearing of HMD with the left lung more opacified
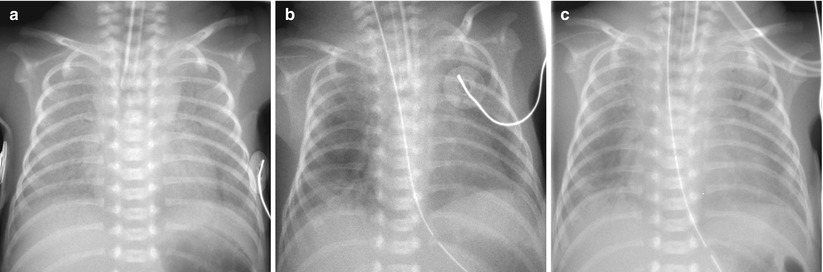
Fig. 11.5
Sudden onset of a white-out has several possible explanations. Pulmonary edema resulted from left-to-right shunting across a patent ductus arteriosus. (a) HMD in a 1-day-old preterm neonate. (b) On the second day of life, there is improvement with residual patchy opacities in the periphery of the lungs. (c) On the third day of life, bilateral diffuse hazy opacification reappeared. Large PDA with left-to-right shunting was confirmed on echocardiography at that time
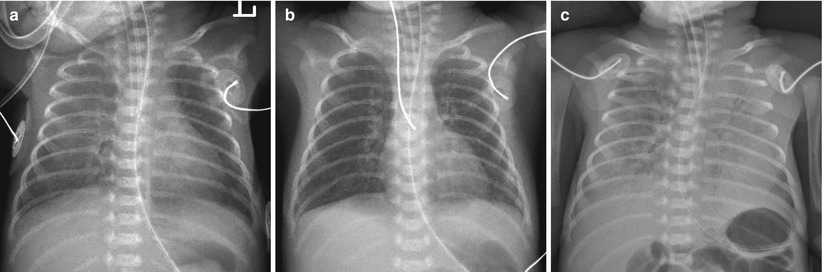
Fig. 11.6
Sudden onset of a white-out has several possible explanations. Pulmonary hemorrhage in a 26-week-gestational-age neonate following surfactant therapy. (a) Frontal chest radiograph obtained after one dose of surfactant shows asymmetric clearing of opacities with the left lung improved more than the right. (b) Radiograph, taken after additional dose of surfactant shows almost clear lungs. (c) Frontal chest radiograph obtained on day 3 of life for evaluation of sudden respiratory compromise and bloody endotracheal aspirates shows diffuse heterogeneous airspace opacifications
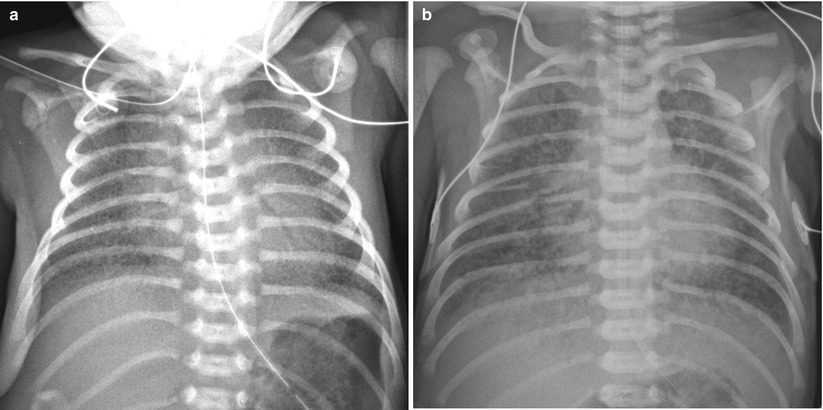
Fig. 11.7
Several diseases may mimic HMD. (a) Group B streptococcal pneumonia in a 34-week-gestational-age newborn. Anteroposterior radiograph shows diffuse granular opacities that look like those of typical HMD. (b) Total anomalous pulmonary venous return (TAPVR) in a 37-week-gestational-age neonate. Anteroposterior radiograph obtained on the first day of life demonstrates bilateral diffuse granular opacities. The lung volume is not small, compared to that of typical HMD. TAPVR with obstruction manifests as pulmonary interstitial infiltrations without significant cardiac enlargement and can appear similar to HMD
11.6.3 Immature Lung Syndrome
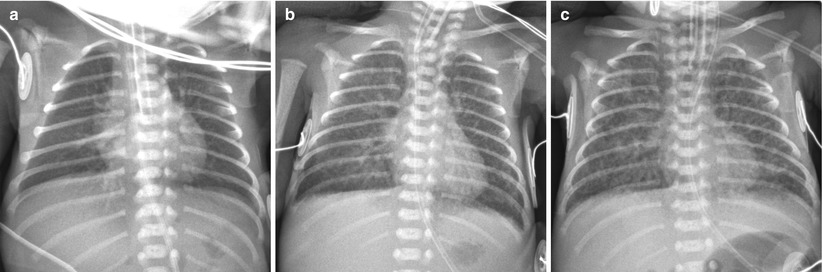
Fig. 11.8
Immature lung syndrome in a profoundly premature neonate delivered at a gestational age of 23 weeks and 6 days with a birth weight of 590 g. (a) Anteroposterior chest radiograph obtained on the first postnatal day shows almost clear lungs and normal lung volume. (b) Follow-up radiograph obtained at 9 days shows diffuse faint haziness with interstitial thickening.(c) Anteroposterior radiograph obtained at 28 days shows a relatively uniform pattern of coarse interstitial opacities with generalized overaeration, which suggests progression to the recent mild form of bronchopulmonary dysplasia
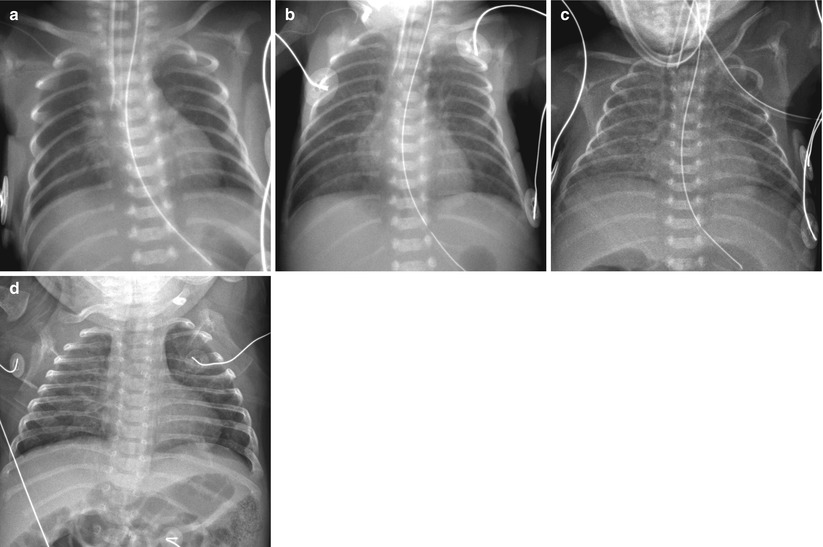
Fig. 11.9
Immature lung syndrome in a neonate born at a gestational age of 23 weeks and 4 days. (a) Anteroposterior radiograph obtained on the day of birth shows ill-defined central lung opacities. (b) At 9 days of age, there is diffuse faint haziness. (c, d) Anteroposterior chest radiographs, obtained at 31 and 90 days, respectively, demonstrate slow evolution to a coarse interstitial pattern of BPD
11.6.4 Bronchopulmonary Dysplasia
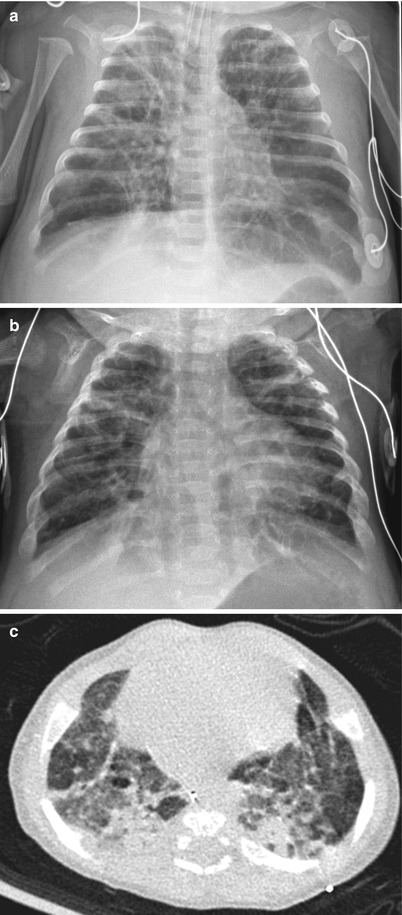
Fig. 11.10
Classic severe bronchopulmonary dysplasia (BPD). (a) Anteroposterior chest radiograph obtained in a preterm infant at 37 weeks’ postmenstrual age shows hyperinflated lungs with alternating areas of lucencies and strand-like coarse opacities. (b, c) Chest radiograph (b) and CT (c) in a 4-month-old infant born at a gestational age of 27 weeks. Chest radiograph reveals diffuse coarse irregular opacities and alternating areas of hyperinflation. Axial CT scan demonstrates lucent areas of variable size, multifocal atelectasis, coarse interstitial lines of fibrosis, and architectural distortion. Bronchiectasis does not occur
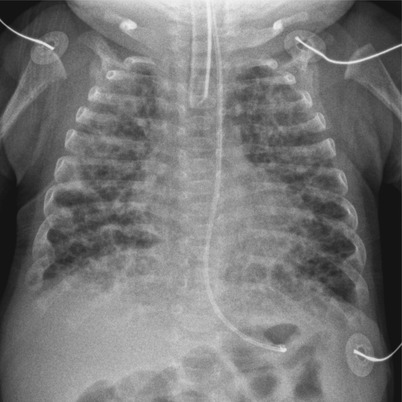
Fig. 11.11
BPD in a 10-week-old infant born at a gestational age of 31 weeks. chest radiograph shows hyperinflation and numerous small round cystic areas alternating with coarse irregular opacities. The findings are more symmetrical and uniform than those seen in Fig. 11.10
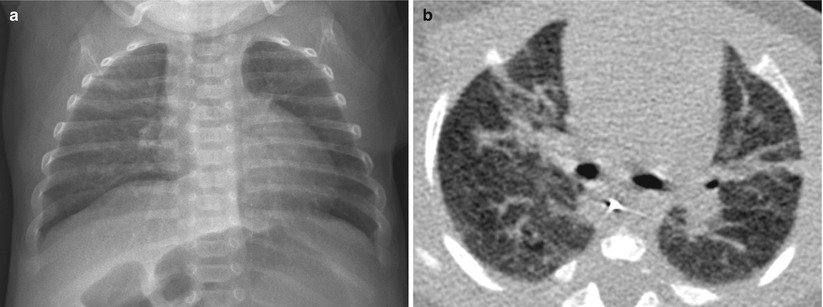
Fig. 11.12
Mild BPD in an 11-week-old infant at a gestational age of 26 weeks. (a) Chest radiograph obtained at the time of discharge shows hyperinflation and fine interstitial opacities. (b) CT performed at the same time shows multifocal linear areas of atelectasis or fibrosis
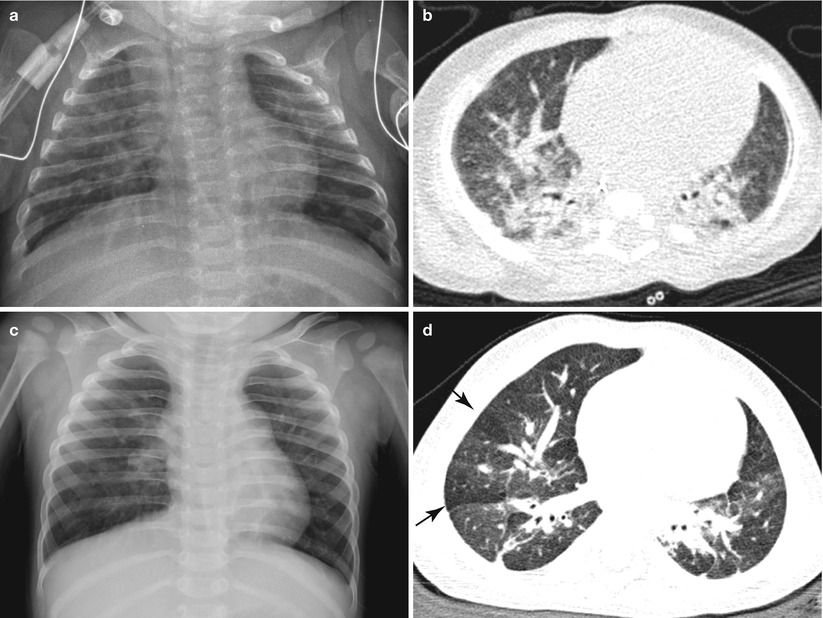
Fig. 11.13
Gradual radiologic improvement of BPD in a pre-term infant born at a gestational age of 30 weeks. (a) Frontal chest radiograph at 3 months of age shows coarse interstitial opacities and overall mild hyperinflation. (b) CT scan obtained at the same time shows multifocal atelectasis and fibrotic opacities, suggestive of BPD. (c) Follow-up chest radiograph taken at 15 months of age reveals interval improvement of interstitial lung opacities, but there are some coarse central interstitial opacities and hyperinflation. (d) On CT image obtained at around the same time, there are improved but residual linear subpleural opacities. Multifocal areas of mosaic attenuation (arrows) can be seen
11.6.5 Meconium Aspiration Syndrome
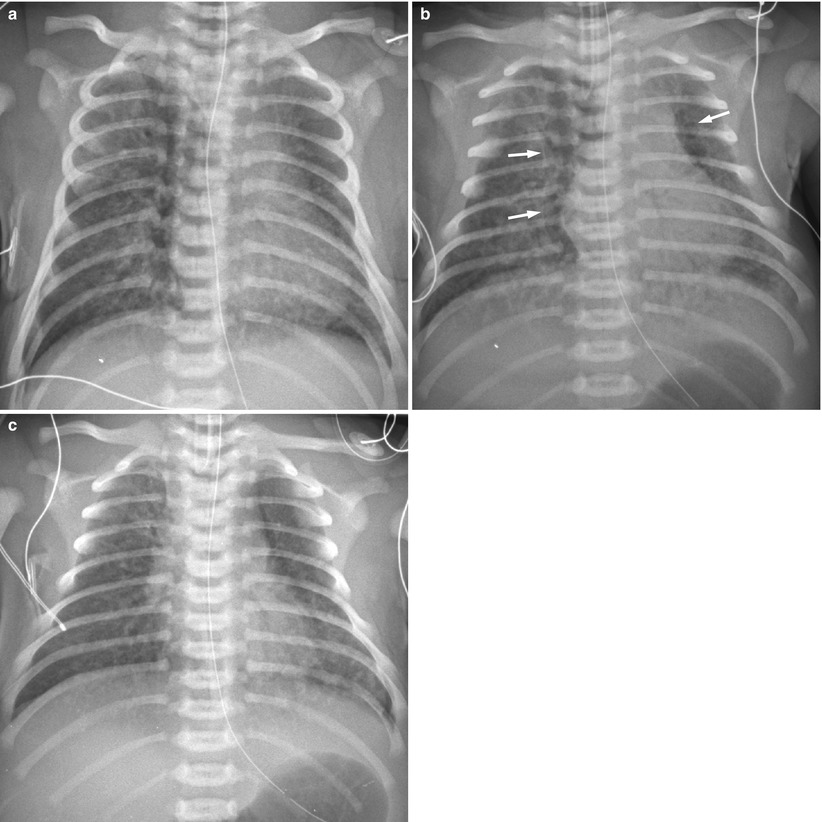
Fig. 11.14
Meconium aspiration syndrome in a 41-week-gestational-age neonate with meconium staining. (a) Anteroposterior chest radiograph demonstrates coarse opacities bilaterally. The lungs are hyperinflated. (b) Repeat radiograph obtained 5 h later reveals pneumomediastinum (arrows). (c) One day later, air leak has improved, but coarse nodular opacities are still noted in both lung fields
11.6.6 Transient Tachypnea of the Newborn
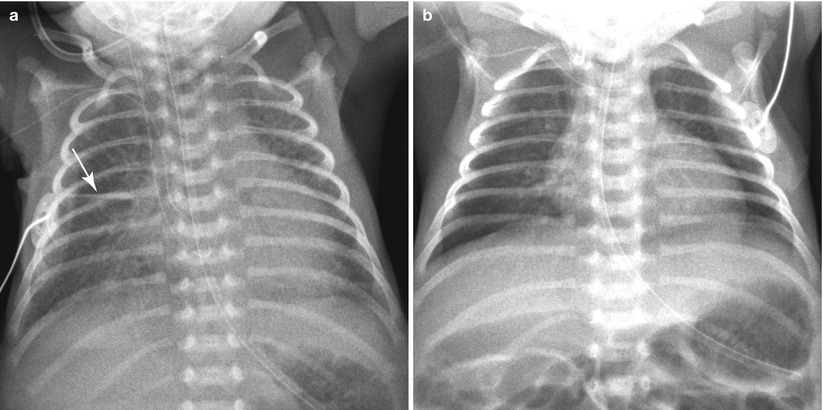
Fig. 11.15
TTN in a 34-week-gestational-age newborn, delivered due to placental abruption. (a) Initial frontal chest radiograph demonstrates cardiomegaly and bilateral parahilar strandings. A small amount of fissural fluid is seen at the right minor fissure (arrow). There was mild respiratory distress, but oxygen saturation was maintained at 95 % in the room air. (b) The opacification has completely cleared on the following day
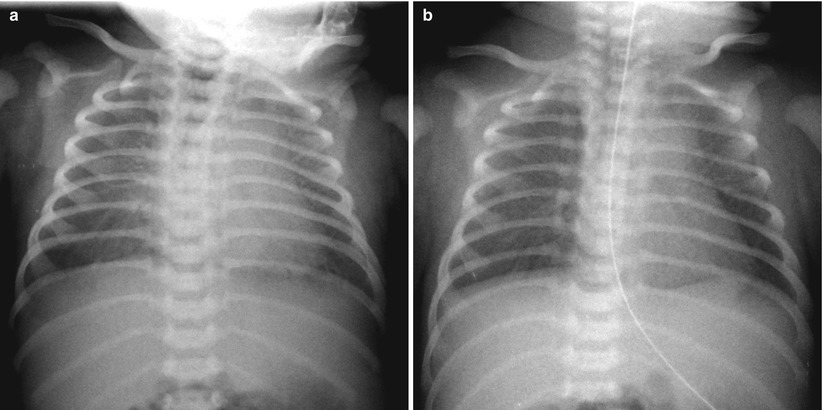
Fig. 11.16
TTN in a 40-week-gestational-age newborn. (a) Bilateral diffuse faint haziness and mild cardiomegaly are noted on a frontal radiograph obtained on the day of birth; tachypnea without cyanosis was present at that time. (b) Such opacification has cleared on repeat radiograph obtained 1 day later. The important diagnostic clues are the patient’s gestational age and rapid resolution of lung lesions
11.6.7 Neonatal Pneumonia
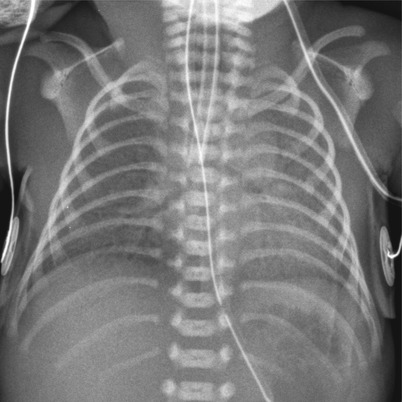
Fig. 11.17
Group B streptococcal pneumonia in a preterm neonate with a history of maternal chorioamnionitis. Anteroposterior chest radiograph reveals bilateral diffuse opacification with air bronchograms. This finding is quite similar to that of HMD
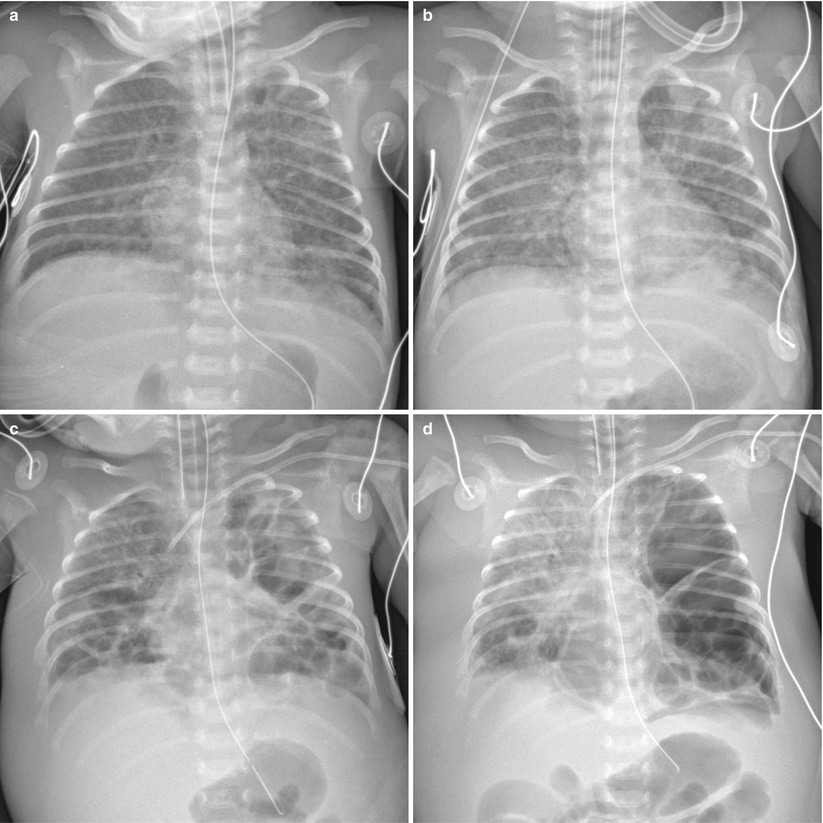
Fig. 11.18




Staphylococcus aureus pneumonia in a 32-week-gestational-age newborn. (a) Anteroposterior radiograph obtained at 10 days of age demonstrates diffuse uniform reticular opacities, suggestive of bronchopulmonary dysplasia. (b) Two days later, follow-up chest radiograph reveals irregular patchy infiltrates in both lungs. From that time, blood cultures were positive for methicillin-resistant Staphylococcus aureus. (c) Anteroposterior radiograph obtained at 19 days of age demonstrates progression into multiple bilateral pneumatoceles. Bilateral pleural effusions and anasarca are also seen. (d) At 24 days, there is progression of the disease. Chest radiograph shows markedly enlarged pneumatoceles in left lung with mediastinal shift to the right
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree