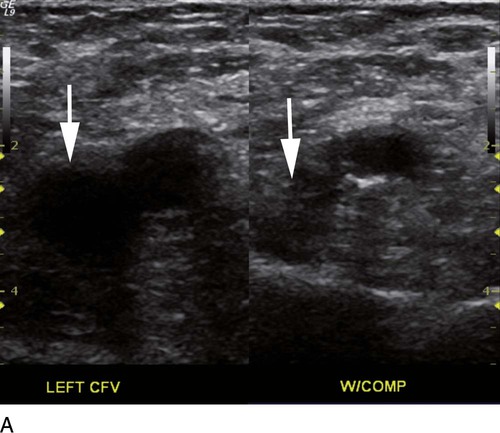
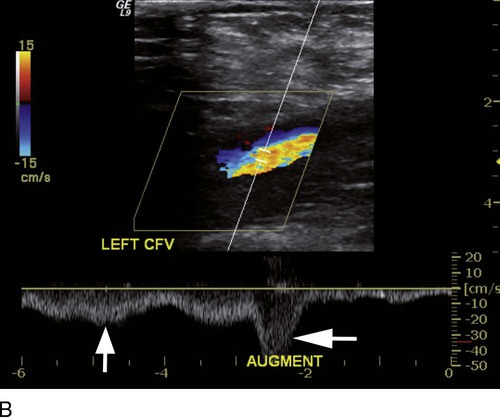
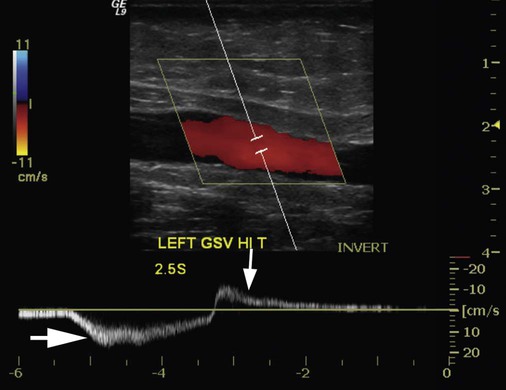
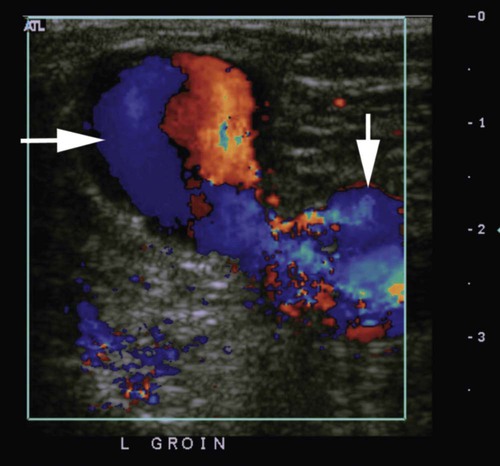
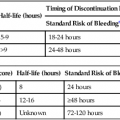
where fD = Doppler shift or Doppler frequency; fR = frequency of the reflected beam; fl is frequency of incident beam, V = velocity of the blood flow, θ = the angle between the direction of flow and the axis of the ultrasound beam, and C = velocity of the sound in tissues. When the angle of interrogation between the incident beam and direction of blood flow approaches 90 degrees, the cos θ approaches 0 and no Doppler shift is detected. In general, the angle of interrogation is kept between 30 and 60 degrees with the vessel lumen to receive reliable velocity measurements. Doppler evaluation of the venous system in lower extremities is usually performed to detect deep or superficial venous thrombosis (compression ultrasonography) or valvular insufficiency.1 Normal patent veins are anechoic and directly compressible with the transducer (Fig. e2-1, A). The Doppler spectrum demonstrates flow toward the heart, with respiratory variations in the flow pattern (the phasicity). Normal phasic flow in the distal veins suggests patent central veins. In addition, augmentation of flow can be demonstrated with distal compression (Fig. e2-1, B). An augmentation response usually suggests patent veins peripherally from the site of Doppler interrogation to the site of compression. Acute deep venous thrombosis (DVT) is predisposed by hypercoagulable states (congenital or acquired), endothelial injury, and stasis of blood flow (Virchow triad). The common predisposing clinical conditions for DVT are malignancy, trauma, perioperative states, and postpartum, congenital, or acquired hypercoagulable states. Ultrasonography is highly sensitive in the diagnosis of acute DVT.2 Acute thrombosis results in an enlarged vein with intraluminal low-level echoes. The vein is noncompressible, and the Doppler spectrum shows no demonstrable flow. Subacute thrombus is seen as moderate high-level echoes in the lumen, with minimal or no enlargement of the vein. Chronic thrombosis is difficult to identify because the veins become small and may sometimes demonstrate channels of flow (partial recanalization of thrombus). Rarely, calcification of the vein may be seen as a focal highly echogenic area with distal acoustic shadowing. Venous insufficiency predisposes to varicose veins, leg edema, venous stasis dermatitis, and venous ulcers. It can occur in the superficial or deep veins. Chronic venous insufficiency affecting the deep veins is usually predisposed by prior DVT. Venous insufficiency affecting the superficial veins may be related to prior thrombosis, but most often no cause is found. The elderly are predisposed to its development, and pregnancy and genetic factors also play a role. To plan appropriate therapy, it is necessary to document the site and degree of venous insufficiency and assess the anatomy of the superficial veins, perforator veins, and their relative contribution to varicosities. Venous insufficiency should ideally be assessed with the patient standing. A competent vein demonstrates flow toward the heart and transient reflux (<0.5 second) on a Valsalva maneuver or after distal compression. Reflux (Fig. e2-2) refers to reversal of flow direction (normal flow direction is toward the heart, from the superficial veins to the deep veins in case of perforator veins) and is graded depending on the length of time the flow reversal is observed.3 A longer duration of reflux implies more severe disease. Other parameters such as reflux velocity and the calculated reflux volume have been used to assess the severity of reflux.4 Ultrasonography may be requested to evaluate groin complications after endovascular interventions. These include hematoma, pseudoaneurysm, arteriovenous fistula, and arterial occlusion. Pseudoaneurysm is diagnosed when an anechoic mass with internal flow is identified adjacent to the puncture site. Generally a communication (i.e., “neck”) is identified between the mass and the artery (Fig. e2-3). If the communication is narrow, the pseudoaneurysm may be treated via ultrasound-guided thrombin injection. Arteriovenous fistula is diagnosed when the artery proximal to the fistula shows monophasic flow with increased diastolic flow, and the vein shows arterialized pulsatile flow. The actual site of communication is rarely seen by ultrasonography and may demonstrate elevated flow velocities. Arterial occlusions are secondary to thrombus formation or dissections. Ultrasonography may show intraluminal low-level echoes without flow on color flow imaging. Renal artery stenosis is an uncommon but correctable cause of hypertension. The underlying etiology may be atherosclerosis (in the elderly), fibromuscular dysplasia (in the young), or may be congenital. Ultrasonography is used as a screening modality with variable success. Sensitivity varies from 0% to 98% among various studies.5,6 There are two approaches for the diagnosis of renal artery stenosis. The extrarenal arteries are evaluated for peak systolic velocity, and a velocity of more than 200 cm/s suggests a stenosis of more than 60% luminal diameter. In addition, the ratio of peak systolic velocity in the main renal artery and peak systolic velocity in the abdominal aorta (referred to as the renal/aortic ratio) is calculated. Renal artery stenosis is suspected if the ratio is more than 3.5. The second approach is to evaluate the intrarenal arteries by assessment of poststenotic flow dynamics. Renal artery stenosis is suspected if the intrarenal arteries demonstrate a tardus parvus pattern with an acceleration time (time to reach peak systolic velocity from baseline) more than 100 ms. Estimating the resistive index (RI) in the intrarenal arteries may be useful because an RI greater than 0.8 suggests a poor chance of recovery from renal dysfunction or hypertension after renal artery angioplasty. The main limitations of ultrasonography are difficulty in identifying the extrarenal arteries, inability to detect accessory renal arteries, and operator dependence. The extrarenal arteries may be better defined on ultrasonography with the use of contrast agents. Patients with chronic mesenteric ischemia present with postprandial pain and weight loss. There are excellent collateral pathways between the celiac axis, superior mesenteric artery, and inferior mesenteric artery. Unless two or more of these arteries are stenotic or occluded, it is rare to develop chronic mesenteric ischemia. A stenosis of more than 70% may be suspected if peak systolic velocities in the celiac axis and superior mesenteric artery exceed 200 cm/s and 275 cm/s, respectively.7 Indirect signs that suggest stenosis include identification of flow reversal in the vessels distal to the stenosis and enlargement of the inferior mesenteric artery. In addition, the normal expected postprandial increase in peak systolic velocity and diastolic flow may not be seen in the presence of severe stenosis of the superior mesenteric artery.8
Noninvasive Vascular Diagnosis
Ultrasonography
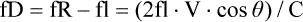
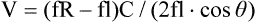
Clinical Applications
Lower Extremities
Veins
Arteries
Abdominal Vessels
Renal Artery
Mesenteric Arteries
Noninvasive Vascular Diagnosis