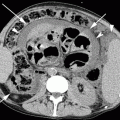
Fig. 40.1
Lateral images obtained during fluoroscopy-guided C2-C3 facet joint injection. (a) An artery forceps is used to determine the desired skin entry site. (b) The needle is in a satisfactory position, projecting over the C2–C3 facet joint. (c) Intra-articular position is confirmed with injection of nonionic contrast. The facet joint is clearly outlined
There are two ways of confirming intra-articular position of the needle tip. The first method is by gently rotating the patient from side to side while observing the position of the needle tip under fluoroscopy. The needle tip should move with the joint in all positions if it is correctly placed within the joint space. Alternatively, a facet arthrogram may be done, and the contrast agent should be seen flowing away from the needle tip extending to the superior or inferior recesses of the joint capsule. If the contrast agent forms a blob at the injection site, the needle is likely to be extra-articular. Usually 0.1–0.2 ml of nonionic contrast agent is sufficient as excessive contrast injection may restrict subsequent drug mixture injection due to limited space within the facet joint.
Once the position of the needle is confirmed, the prepared drug mixture is then injected. Extra care should be taken to prevent moving the needle while engaging the syringe. There should be no or minimal resistance to injection if the needle tip is within the facet joint.
Alternatively, the procedure may be performed using a posterior approach (Silbergleit et al. 2001). The patient is placed in the supine oblique position at approximately 45° with the side to be injected facing up. The head should be turned away from the side to be injected. The frontal tube is then angled until the facet joint is best visualized, and a 22-G needle is then advanced into the joint. True lateral and anteroposterior views are obtained subsequently to confirm the needle position.
40.2.5.2 Thoracic Facet Joint Injection
The thoracic facet joints are nearly vertical and coronal in orientation, with a gradual change from coronal to sagittal orientation at the thoracolumbar junction (Eckel 2004). The facet joints are inclined nearly 60° to the coronal plane and rotated so that the superior facet faces posteriorly, superiorly, and laterally (Sohaib and Butler 1999). The lateral aspect of the joint is located anteriorly with the medial side in a more posterior location.
The general principles and equipment used for thoracic facet joint injection are similar to that of cervical facet joint injection. The main difference is the positioning of the patient, which is prone in the case of thoracic facet joint injection. The medial side of the joint has a more posterior location and is therefore more superficial than the lateral half when the joint is targeted through posterior approach. This explains why the medial half of the joint is the preferred target point in our practice.
Once the skin is prepared and draped, the vertebral levels are confirmed using fluoroscopic guidance. The point of skin entry is marked at the middle of the pedicle of the vertebra one level below the facet joint being injected (i.e., if T6–T7 facet joint is targeted, the skin entry site should be marked at the middle of the pedicle of T8 at the same side). The needle is angled at a 60° angle at the puncture site and advanced cranially towards the targeted facet joint under fluoroscopic guidance. The track of the needle is maintained between an imaginary plane along the medial and lateral borders of the pedicle to avoid inadvertent puncture of the thecal or the lungs. When the needle tip reaches the upper border of the pedicle of the vertebra one level above the skin entry site, the C-arm is then rotated 80–90° towards the lateral projection to visualize the target joint in profile and determine the depth of the needle in relation to the target facet joint. The needle tip should be seen just below the posteroinferior aspect of the target joint, where further advancement of the needle would enter the facet joint.
In view of the steep angle of entry and overlapping of bony structure that could make accurate assessment of the needle position somewhat more difficult, injection of a small amount of contrast agent is recommended to confirm the intra-articular position of the needle when the thoracic facet joints are targeted. In difficult cases, for example, patients with extensive degenerative changes or distorted anatomy due to severe scoliosis, periarticular injection may be performed. Alternatively, the procedure can be done under CT guidance, which is actually the preferred method in our institution.
For CT-guided injection, the procedure can be performed by a posterolateral approach. Due to the coronal orientation of the thoracic facet joint, it is not possible to position the needle tip into the interfacetal portion of the joint. Aim to place the needle tip just below the inferior margin of the inferior articular facet and posterior to the superior articular facet. This allows penetration of the inferior joint recess, thus allowing injection into the joint space (Czervionke and Fenton 2003). The technique described by Czervionke and Fenton uses a coaxial needle approach. An 18-G, 3.5-in. spinal needle is placed by a posterolateral approach so that the tip of the needle is 5–10 mm posterior and lateral to the facet joint. After removal of the stylet, a longer 22- or 25-G pre-curved (10–20°) spinal needle is then placed through the 18-G needle in a coaxial fashion to access the ventrolateral aspect of the joint space.
40.2.5.3 Lumbar Facet Joint Injection
The lumbar facet joints are curved structures with a sagittal oblique orientation. The superior facets face anterolaterally, while the inferior facets face posteromedially (Hadley 1961; Selby and Paris 1981). Hence, the posterior aspect of the joint lies further away from the midline than the anterior aspect.
The procedure may be performed under fluoroscopy or CT guidance, with the latter preferred in difficult cases. The patient lies prone in both settings with a pillow placed under the abdomen to reduce lumbar lordosis. During posteroanterior fluoroscopy, the patient is gently rotated through a sideways arc, and the first portion of the joint seen in profile will be its posterior aspect. Further rotation will bring the anterior aspect into profile. It is therefore important not to over-rotate the patient as the anterior portion of the joint space may come into view rather than the posterior portion. This will make needle placement into the joint impossible. The upper lumbar spine may require obliquity of as little as 30°, while the lower lumbar spine generally requires more obliquity of up to 60°.
When the joint space is visualized with least obliquity, its location is marked on the skin. Following standard skin preparation and local anesthetic injection, a 22-G spinal needle is directed vertically into the center of the facet joint space (Fig. 40.2). Direction of the needle should be checked intermittently during advancement. The intra-articular position of the needle tip can be confirmed with either of the two methods described in the cervical facet joint section. Injection should be resistance-free if the needle tip is within the facet joint.


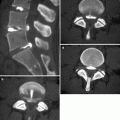
Fig. 40.2
(a) Fluoroscopic image shows the needle tip projected over the left L3/L4 facet joint. (b) Nonionic contrast injection confirmed intra-articular position of the needle. The inferior recess is also opacified (arrow)
40.2.5.4 Periarticular Injection
If there is difficulty getting into the facet joint proper, for example, in patients with large obstructing osteophytes, periarticular injection is an acceptable alternative (Lilius et al. 1989; Gopinathan and Peh 2011). During a periarticular injection, the needle is rotated 360° at the desired location, and 1.0–1.5 ml of the local anesthetic and steroid mixture is injected around the facet joint. The injection is terminated when resistance is encountered.
40.2.6 Post-procedure Care and Complications
The patient should be monitored for at least 15 min prior to discharge (Gorbach et al. 2006). Check the puncture site for any hematoma. The patient should be instructed to report any acute worsening of existing neck or back pain or development of any neurological symptoms, for example, numbness or weakness of extremities. Driving is not advisable in patients who develop such symptoms after the procedure. Simple oral analgesics can be prescribed if patients experience pain at the site of injection.
Complications of facet joint injection are rare, particularly if the procedure is performed using a precise needle positioning technique (Gorbach et al. 2006; Sehgal et al. 2007). Possible complications can be classified into those related to needle placement and drug administration.
Complications related to needle placement include bleeding, spondylodiscitis, septic arthritis, dural puncture, spinal cord injury, intravascular injection, spinal anesthesia, neural trauma, and pneumothorax (Manchikanti et al. 2003; Falagas et al. 2006; Weingarten et al. 2006; Gopinathan and Peh 2011). Postinjection anesthesia and paralysis are usually transient and expected to resolve within minutes to hours. A case of transient tetraplegia after cervical facet joint injection has been previously reported by Heckmann et al. (2006), but the procedure was performed without imaging guidance. Isolated cases of epidural and spinal abscesses (Cook et al. 1999; Alcock et al. 2003; Gaul et al. 2005), meningitis (Gaul et al. 2005), and generalized infection leading to death (Kim et al. 2010) have also been reported. This emphasizes the importance of strict adherence to an aseptic technique.
Severe allergic reactions to contrast agent and local anesthetic are uncommon. Steroid injection may produce a local reaction, most often occurring immediately after injection and may last for up to 48 h (Gopinathan and Peh 2011). Ice packs help to relieve the symptoms. Systematic side effects of steroids are usually not an issue, given the small dose and localized injection, unless there are repeated injections at multiple levels.
40.2.7 Results
The diagnostic accuracy of imaging-guided facet joint injections is strong in the diagnosis of facet joint-related neck and low back pain and moderate evidence in the diagnosis of pain arising from thoracic facet joints (Sehgal et al. 2007). With regard to its therapeutic efficacy, most of the available data are based on noncontrolled and observational studies. Only a few exhaustive randomized control trials are available, mostly pertaining to the lumbar spine. Short-term relief of symptoms (1–4 weeks) after lumbar facet injections was observed in 42–92 % of patients (Lynch and Taylor 1986; Carette et al. 1991). Medium-term relief at 3 months ranges from 18 to 62 % (Lynch and Taylor 1986; Marks et al. 1992). A systematic review by Boswell et al. (2007) concluded that for cervical intra-articular facet joint injections, the evidence is limited for short- and long-term pain relief. For lumbar intra-articular facet joint injections, the evidence is moderate for short- and long-term pain relief.
40.3 Sacroiliac Joint Injection
Pain originating from the sacroiliac joint is often a clinically difficult diagnosis due to the lack of specific clinical signs and rather poor correlation with radiologic findings (Chou et al. 2004). The pain pattern may cover the lower lumbar region, buttocks, hip, or proximal thigh, mimicking pain referred from prolapsed lumbar intervertebral disc, lumbar facet joints, and also the hip. However, patients with sacroiliac joint pain do not have neurological deficits, in contrast to those with nerve root compression. Dreyfuss et al. (1996) found that none of the 12 recommended sacroiliac joint tests or any historical feature is consistently capable of identifying dysfunctional sacroiliac joint as the source of pain.
Similar to the facet syndrome, the gold standard in confirming sacroiliac-related low back pain is by reproduction of pain through intra-articular injection of saline or contrast agent into the joint, with subsequent relief of pain after local anesthetic injection. This has been demonstrated by Haldeman and Soto-Hall (1938) and Fortin et al. (1994), when they injected procaine and saline, respectively, into the sacroiliac joints. Known causes of sacroiliac-related low back pain include spondyloarthropathy, infections, metabolic diseases, trauma, osteitis condensans ilii, and idiopathic sacroiliitis (Levin et al. 2009).
40.3.1 Anatomy
The sacrum consists of five fused vertebrae that are in continuity with the lumbar spine proximally and coccyx inferiorly. It articulates with the two ilia laterally, forming the sacroiliac joints. The sacroiliac joint represents the largest axial joint in the body, with an average surface area of 17.5 cm2 (Cohen 2005). Although characterized as a diarthrodial synovial joint, only the anterior third of the interface between the sacrum and ilium is a true synovial joint. The posterior two-thirds are comprised of an intricate set of ligamentous connections functioning to reduce movement of the joint (Cohen 2005). Unlike the lumbar facet joints, the sacroiliac joint lacks a well-defined capsule. The anterior joint capsule is thickened and the posterior capsule is either absent or rudimentary. No consensus has been reached with regard to the innervations of the sacroiliac joints. A review by Fortin et al. (1999) concluded that the joint is predominantly innervated by the sacral dorsal rami.
40.3.2 Indications
Current indications for sacroiliac joint injections are:
1.
Low back and lower extremity pain below the level of L5
2.
Lack of obvious evidence for disc-related or facet joint pain
3.
Failure to respond to more conservative management such as physiotherapy, chiropractic management, or analgesics
40.3.3 Contraindications
1.
Ankylosed sacroiliac joints
2.
Other contraindications are similar to those for facet joint injection
40.3.4 Technique
The procedure is usually done as outpatient basis, with no specific pre-procedural preparation or premedication required. It may be performed under fluoroscopic or CT guidance, with the latter being the preferred method in our institution due to the rather complex configuration of the sacroiliac joint. Ultrasound-guided sacroiliac joint injection technique has also been described previously (Pekkafahli et al. 2003; Harmon and O’Sullivan 2008).
The patient is placed in the prone position. The sacral and buttock area is cleaned and draped using an aseptic technique. On fluoroscopy, the frontal projection will show two apparent joint spaces (Fig. 40.3). The more medially located joint space is the lower synovial portion of the true sacroiliac joint, whereas the upper fibrous portion of the joint is projected more laterally (Levin et al. 2009). It is the inferior joint space that is targeted during sacroiliac joint injection. Various techniques of fluoroscopy-guided sacroiliac joint have been described. Levin et al. (2009) used rotation of the fluoroscope away from the site of injection in the transverse plane to visualize the inferior joint space, before advancing the needle towards the joint in a medial to lateral direction. Dussault et al. (2000) suggested tilting the fluoroscope about 20–25° in a cephalic direction to displace the posteroinferior portion of the sacroiliac joint in a caudal direction, separating it from the anterior fibrous portion of the joint, which would then move cephalad on the image. Once the skin entry site is confirmed and marked, local anesthetic is given. A 22-G spinal needle is advanced towards the posteroinferior portion of the joint perpendicular to the fluoroscopic table under fluoroscopic guidance.


Fig. 40.3
Frontal radiograph of the sacroiliac joints. Two apparent joint spaces are seen. The synovial portion of the joint is more inferior and medially located (arrow). The fibrous portion of the joint is projected more superior and laterally (curved arrow)
Once the needle is correctly placed, its position can be checked by moving the fluoroscopy tube in the right and left oblique positions under continuous fluoroscopy. If the needle tip is within the sacroiliac joint, the needle tip should remain within the joint in all projections and not projected over the bone. Alternatively, a small amount (usually no more than 0.5 ml) of low osmolar nonionic contrast agent can be injected to confirm the intra-articular position. The injected contrast agent should outline the joint space if the needle tip is within the joint. Upon confirmation of position, 3–5 ml of the local anesthetic and steroid mixture (similar to the mixture used for facet joint injection) is injected.
It is more straightforward if the procedure is performed under CT guidance, as the position of the needle tip can be clearly visualized. Another advantage of CT guidance is the ability to identify the sciatic nerve and the adjacent gluteal artery, which should be avoided during needle advancement. Similar to fluoroscopically guided injection, the patient again adopts a prone position, and the poster inferior portion of the sacroiliac joint is targeted (Fig. 40.4).


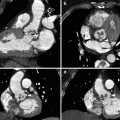
Fig. 40.4
Sacroiliac joint injection in a 49-year-old woman with bilateral sacroiliac joint pain. (a) Axial CT image depicts needle tip (arrow) within the left gluteus muscle, directed towards the left sacroiliac joint. (b) Subsequent image shows needle tip placement (arrow) within the posterior left sacroiliac joint, ready for injection. The right sacroiliac joint is fused and hence was not injected (curved arrow)
40.3.5 Post-procedure Care and Complications
Standard post-procedure care, as mentioned in the facet joint injection section, is followed. The post-procedure caregiver should ask specifically for lower limb neurological symptoms which can occur after inadvertent sciatic nerve injury. No serious complications after sacroiliac joint injection, related to either needle placement or drug injection, have been reported.
40.3.6 Results
Both observational studies and randomized controlled trials have found imaging-guided sacroiliac joint injection to be effective in providing significant pain relief. Positive response is achieved in 80–90 % of patients, with the pain relief effect lasting up to several months (Bollow et al. 1996; Maugars et al. 1996; Dussault et al. 2000; Slipman et al. 2001; Karabacakoglu et al. 2002). Recurrence of pain is not uncommon and usually occurs after 6–12 months (Hanly et al. 2000). In such cases, repeated injection has been shown to be beneficial (Gunaydin et al. 2006). In difficult cases, periarticular sacroiliac joint injection may be of some value (Luukkainen et al. 1999; Luukkainen et al. 2002).
40.4 Hip Joint Injection
The hip joint is yet another common source of chronic pain in the elderly, primarily due to degenerative osteoarthritis. Other causes include secondary osteoarthritis due to severe avascular necrosis. Unfortunately, there is no treatment available at the present time to prevent or reverse the degenerative changes that have taken place. Other causes of hip pain also include rheumatoid arthritis and secondary osteoarthritis due to avascular necrosis. The main aim of current treatment is to alleviate symptoms and improve the quality of life of the patient. In patients who are not keen or are unfit for total hip replacement, intra-articular injection of viscosupplements and corticosteroids serves as an alternative treatment. Radiographic severity of osteoarthritis does not seem to correlate with severity of symptoms and response to injection (Plant et al. 1997; Robinson et al. 2007).
40.4.1 Technique
Unlike the knee joint, the hip joint is more deeply situated and cannot be easily palpated, making accurate intra-articular injection a challenging procedure. The success rate and safety of intra-articular injection without imaging guidance have been reported to be suboptimal in a cadaveric study performed by Leopold et al. (2001). Depending on the experience of the procedurist and availability of imaging facilities, hip injection can be performed under fluoroscopy, ultrasound, or CT guidance. Compared to fluoroscopy, ultrasound and CT enable clear visualization of the important femoral neurovascular bundle. Ultrasound also has the advantages of easy accessibility, compact size, and lack of radiation exposure, but it is also more user dependent.
Fluoroscopy-guided hip injection is performed with the patient placed in the supine position with the foot pointing upwards. An aseptic technique is applied. An anteroposterior approach, under fluoroscopic guidance, is the most commonly used method. The femoral pulse should always be palpated and a marker placed over the pulse to avoid inadvertent femoral vessels and nerve injury. As the hip joint has a large joint capsule that covers up to the mid-femoral neck region, there is a relative large area that could be targeted as needle placement anywhere within the capsule will ensure intra-articular injection. The lateral aspect of the proximal femoral neck at the head-neck junction (Fig. 40.5) is a good needle placement point since it is well within the coverage of the joint capsule and minimizes the risk of femoral vessel and nerve injury.


Fig. 40.5
Frontal images obtained during fluoroscopic-guided intra-articular left hip joint injection. (a) Artery forceps acts as a landmark of the femoral artery. The lateral aspect of the femoral head-neck junction is targeted using a 22-G spinal needle. (b) Injection of nonionic contrast outlines the joint capsule (arrows), indicating intra-articular position of the needle
Once the skin entry site is identified and local anesthetic infiltrated, a 22-G spinal needle is advanced towards the target point on the bone at a cranially oblique angle to the skin. When the needle touches the bone, intra-articular position of the needle tip should then be confirmed with injection of 3–5 ml of contrast agent. The joint capsule should be outlined if the needle tip is within the joint capsule. Corticosteroid injection then can be performed safely.
In our institution, injection of hip joint is performed under CT guidance if a viscosupplement is injected, due to its potential to react with the contrast agent. If CT guidance is used, a preliminary axial scan is performed to determine the skin entry point. The femoral neurovascular bundle is identified, and the skin entry point is lateral to it (Fig. 40.6). Upon confirmation of the entry point, the needle is directly advanced into the inferior part of the hip joint.


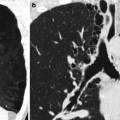
Fig. 40.6
CT-guided intra-articular injection of the right hip. (a) The femoral neurovascular bundle (arrow) is identified and the needle entry point is located laterally to it. (b) The needle tip is well within the right hip joint, ready for injection of the viscosupplement
40.4.2 Post-procedure Care and Complications
Mild pain or discomfort after injection is not uncommon and can be managed with simple oral analgesics. The site of injection should be monitored for any hematoma secondary to inadvertent femoral vessel puncture. If this occurs, sustained firm manual compression is usually effective. Other reported but rare complications include septic arthritis, osteonecrosis, and joint infection after total hip replacement following preoperative intra-articular injection (Chazerain et al. 1999; Nallamshatty et al. 2003; Yamamoto et al. 2006




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree