LEARNING OBJECTIVES
1. Recognize a pattern of peripheral lung disease on chest radiography or computed tomography (CT) and give an appropriate differential diagnosis, including a single most likely diagnosis when supported by associated radiologic findings or clinical information (e.g., peripheral lung disease associated with paratracheal and bilateral hilar lymphadenopathy in an asymptomatic patient with alveolar sarcoidosis; peripheral lung disease associated with a markedly elevated blood eosinophil count in a patient with eosinophilic pneumonia; peripheral opacities associated with multiple rib fractures and pneumothorax in a patient with acute chest trauma and pulmonary contusions; and multiple peripheral wedge-shaped opacities associated with pulmonary emboli in a patient with multiple pulmonary infarcts).
2. Name four predisposing conditions that lead to cryptogenic organizing pneumonitis.
The diseases in this chapter are not ordinarily discussed together. Alveolar sarcoidosis, usual interstitial pneumonias, and contusions are, in fact, discussed in Chapters 10, 3, and 8, respectively. Cryptogenic organizing pneumonia (COP) (previously referred to as bronchiolitis obliterans organizing pneumonia) and eosinophilic pneumonia (EP) could be discussed in a chapter on diffuse interstitial lung disease. Pulmonary infarcts are discussed with pulmonary emboli in Chapter 17. However, these disorders have been categorized together here because of their propensity for producing a predominantly peripheral distribution of disease on chest radiography and computed tomography (CT). A simple mnemonic, “AEIOU,” can be used to remember those disorders that have a peripheral distribution of disease (Table 12.1 and Figs. 12.1 and 12.2). It needs to be mentioned, however, that these disorders frequently do not manifest as recognizable peripheral opacities on chest radiography or CT, so a lack of peripheral opacities does not exclude these disorders. If there is any hint of a peripheral distribution of disease on the chest radiograph, CT can be very helpful in better defining the morphology and distribution of disease (Fig. 12.3).
Table 12.1 DISORDERS FREQUENTLY MANIFESTING AS PERIPHERAL OPACITIES ON CHEST RADIOGRAPHY OR COMPUTED TOMOGRAPHY
“AEIOU”
Alveolar sarcoidosis
Eosinophilic pneumonia
Infarcts
COP (cryptogenic organizing pneumonia)
UIP (usual interstitial pneumonitis and DIP [desquamative interstitial pneumonitis])
ContUsions
ALVEOLAR SARCOIDOSIS
Sarcoidosis is a systemic disease of unknown etiology that is characterized by widespread development of noncaseating granulomas. More complete discussions of this disorder are found in Chapters 3, 6, and 10. This section will be limited to a discussion of those patients with sarcoidosis who have so-called “alveolar” opacities on chest radiography or CT.
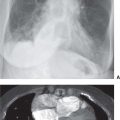
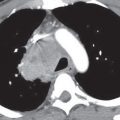
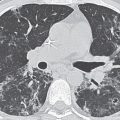
Although the term alveolar sarcoidosis is used, the process is predominantly interstitial, with compression and obliteration of alveoli creating the appearance of alveolar filling on radiologic imaging. Histologically, these lesions are seen to represent confluent interstitial granulomas. The radiographic appearance of airspace (alveolar) opacities develops in 10% to 20% of patients with sarcoidosis. The radiologic appearance is that of bilateral, multifocal, poorly defined opacities that range in size from 1 to 10 cm and show a predilection for the peripheral midlung, sparing the costophrenic angles (1–3) (Fig. 12.4). The peripheral distribution is better seen and on occasion appreciated only on CT. Air bronchograms are common. Associated CT findings of reticulonodular opacities, especially in a perilymphatic distribution, and mediastinal and hilar lymphadenopathy provide clues to the correct diagnosis. Most patients with alveolar sarcoidosis have accompanying lymphadenopathy (4) (Fig. 12.5). When only peripheral opacities are seen, the appearance can be indistinguishable from those of COP or EP. Although all three disorders can present with blood eosinophilia, the degree of eosinophilia is most pronounced with EP. The peripheral opacities of alveolar sarcoidosis can clear rapidly with or without steroid treatment (1).

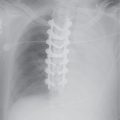
FIG. 12.1 • Usual interstitial pneumonia. A: Posteroanterior (PA) chest radiograph of a 72-year-old woman with scleroderma shows low lung volumes and bilateral reticular interstitial lung disease. B: CT shows that the reticular opacities have a subpleural, peripheral distribution (arrows).

FIG. 12.2 • Usual interstitial pneumonia. A: PA chest radiograph of a 58-year-old woman shows bilateral, subpleural, reticular interstitial lung disease. B: CT shows bilateral, subpleural honeycombing and traction bronchiectasis.

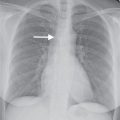
FIG. 12.3 • Eosinophilic pneumonia. A: PA chest radiograph of a 21-year-old woman shows bilateral airspace opacities that extend to the lung periphery. B: CT better shows the peripheral distribution of disease. Note the prominent air bronchograms.

FIG. 12.4 • Alveolar sarcoidosis. A: PA chest radiograph of a 28-year-old asymptomatic man shows nonsegmental, peripheral airspace disease and bilateral hilar and mediastinal lymphadenopathy. B: CT shows bilateral, peripheral airspace disease. Note the air bronchograms (arrows), a common feature of alveolar sarcoidosis.

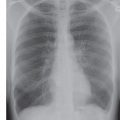
FIG. 12.5 • Alveolar sarcoidosis. A: PA chest radiograph of a 29-year-old man shows ill-defined opacities in the upper lungs (circles). B: CT shows peripheral airspace disease. Note the nodular beading of a left lower lobe bronchovascular bundle (arrow), a characteristic feature of sarcoidosis. C: CT with mediastinal windowing shows bilateral hilar (arrows) and subcarinal (asterisk) lymphadenopathy. Most patients with alveolar sarcoidosis have accompanying lymphadenopathy.
EOSINOPHILIC PNEUMONIA
The term pulmonary eosinophilia, synonymous with pulmonary infiltration with eosinophilia, describes a group of diseases in which blood and/or tissue eosinophilia affects major airways and lung parenchyma (5). Blood eosinophilia, however, is not necessary to make a diagnosis of eosinophilic lung disease. The number of diseases included under the umbrella term pulmonary eosinophilia are numerous. A simple mnemonic, “NAACP,” and the term idiopathic can be used to remember the major classifications of disorders that constitute pulmonary eosinophilia (Table 12.2). This section will focus on parasitic infections and idiopathic pulmonary eosinophilia.
Tropical pulmonary eosinophilia is a systemic disease caused by hypersensitivity to microfilariae, which are the early larval forms of various filarial nematodes, most notably Brugia malayi and Wuchereria bancrofti (6). The disease is endemic in the Indian subcontinent, Southeast Asia, the South Pacific, North Africa, and South America. In nonendemic areas, the disease is seen in immigrants. The predominant respiratory symptom is chronic cough, which is often worse at night. Patients have marked blood eosinophilia, elevated immunoglobulin E (IgE) levels, and a high titer of antifilarial antibody. The chest radiograph is abnormal in the majority of patients, with the most common abnormality being fine linear opacities that are distributed diffusely and symmetrically. Small nodules, ranging in size from 1 to 5 mm, are seen in about half of cases. Hilar lymphadenopathy is uncommon and, when present, is mild (7). An important diagnostic criterion is rapid response to treatment with diethylcarbamazine. Chronic interstitial fibrosis can develop in some patients (8).
The larval stages of a number of worms other than filarial nematodes can pass through the lung and cause EP. These include Ascaris lumbricoides, Strongyloides stercoralis, Toxocara canis, Trichuris trichiura, and Schistosoma sp. The radiologic pattern is usually identical to that of acute EP.
Table 12.2 CLASSIFICATION OF EOSINOPHILIC LUNG DISEASE
“NAACP” and Idiopathic
Neoplasms
Lung cancer
Metastases
Lymphoma
Asthma
Allergic disorders
Allergic bronchopulmonary aspergillosis
Drug-induced disease
Extrinsic allergic alveolitis (hypersensitivity pneumonitis)
Collagen vascular and granulomatous disorders
Rheumatoid lung disease
Churg–Strauss syndrome
Granulomatosis with polyangiitis (Wegener granulomatosis)
Sarcoidosis
Parasitic disorders and other infections
Tropical pulmonary eosinophilia
Helminth infections (worm infestation)
Fungal infections
Other bacterial, viral, and protozoal infections
Idiopathic
Acute eosinophilic pneumonia (Löffler syndrome)
Chronic eosinophilic pneumonia
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree