LEARNING OBJECTIVES
1. Name the four major histologic types of lung cancer and describe the difference between small-cell and non–small-cell types.
2. Name the type of non–small-cell lung cancer (NSCLC) that most commonly cavitates.
3. Name the types of lung cancer that are usually centrally located.
4. Describe the TNM (tumor-node-metastases) classification for staging NSCLC, including the components of each stage (I, II, III, IV, and substages), and define each component (T1 to T4, N0 to N3, M0 to M1).
5. Describe the staging of small-cell lung cancer.
6. Name the four most common extrathoracic sites of metastases for non-small-cell and small-cell lung cancer.
7. Name the stages of NSCLC that are potentially resectable.
8. Recognize abnormal contralateral mediastinal shift on a postpneumonectomy chest radiograph and state five possible etiologies for the abnormal shift.
9. Name the most common thoracic locations for mucoepidermoid, adenoid cystic, and carcinoid tumors to occur.
10. Describe the role of magnetic resonance imaging (MRI) in lung cancer staging (e.g., chest wall invasion and brachial plexus involvement).
11. Describe the role of positron emission tomography in lung cancer staging.
Lung cancer is the third most common cancer and the leading cause of cancer death in the United States (1). The most important risk factor for lung cancer is cigarette smoking, which results in approximately 85% of all lung cancer cases in the United States (Fig. 15.1) (2,3). This chapter will focus on the clinical presentation, histologic classification, and staging of lung carcinoma; this is followed by a brief discussion of lung cancer screening recommendations, postpneumonectomy complications, and carcinoid and salivary gland tumors of the trachea and bronchi.
LUNG CANCER
Clinical Presentation
Lung cancer is relatively uncommon in patients under the age of 30 and typically occurs in 60- to 70-year-old men and women. Patients commonly present with symptoms produced by the primary tumor. Centrally located tumors can cause coughing, wheezing, hemoptysis, and postobstructive pneumonia. Tumors invading the chest wall, pleura, and mediastinal structures can cause pleuritic or local chest pain, dyspnea, cough, the Pancoast syndrome, the superior vena cava syndrome, or hoarseness (from involvement of the recurrent laryngeal nerve). Symptoms can also be related to local or distant metastases (Table 15.1) or paraneoplastic syndromes (systemic manifestations of the primary tumor unrelated to distant metastases). Paraneoplastic syndromes can cause cachexia of malignancy, digital clubbing and hypertrophic osteoarthropathy, nonbacterial thrombotic endocarditis, migratory thrombophlebitis, and various neurologic and cutaneous syndromes. Paraneoplastic syndromes may also be secondary to secretion of ectopic hormones by tumor cells, which can cause hypercalcemia, the syndrome of inappropriate secretion of antidiuretic hormone, Cushing syndrome from corticotropin secretion, gynecomastia, and acromegaly (4).
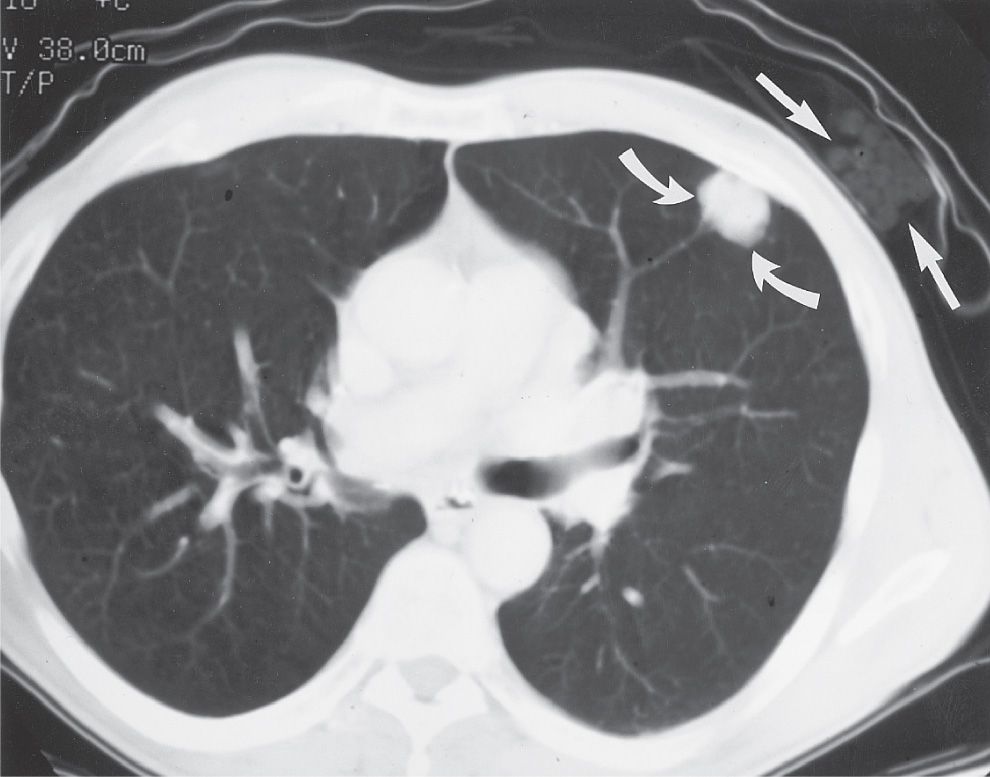
FIG. 15.1 • “Direct link” between cigarette smoking and the development of lung cancer. Note the package of cigarettes within the patient’s shirt pocket (straight arrows), adjacent to the peripheral adenocarcinoma within the left upper lobe (curved arrows).
Histologic Classification
In 2004, the World Health Organization updated its classification of lung tumors on the basis of histologic features (5) and in 2011, the International Association for the Study of Lung Cancer, the American Thoracic Society, and the European Respiratory Society revised the International Multidisciplinary Classification of Lung Adenocarcinoma (6). Four cell types account for more than 95% of all primary lung neoplasms: (i) adenocarcinoma, (ii) squamous cell carcinoma, (iii) large-cell carcinoma, and (iv) small-cell carcinoma. Mixtures of these cell types may occur within the same primary neoplasm, and some tumors are too poorly differentiated to be further classified. Rapid growth, early metastatic spread, and responsiveness to chemotherapy and radiation therapy distinguish small-cell carcinoma from the others, which has led to the classification of “small-cell” and “non–small-cell” carcinoma. Features of the four histologic types are outlined in Table 15.2.
Adenocarcinoma
Adenocarcinoma accounts for 50% of all lung cancers (7), and it is the most common cell type seen in women and nonsmokers. There is a weak association with cigarette smoking and the development of adenocarcinoma. Microscopically, adenocarcinomas are characterized by the formation of glands and papillary structures. Adenocarcinomas can arise from preexistent lung scars, or they can engulf preexisting scars, giving rise to the term scar carcinoma. Like most lung cancers, adenocarcinomas occur most frequently in the upper lobes. They are typically peripheral and subpleural in location, associated with retraction of the adjacent pleura, but can also occur centrally (Figs. 15.2 to 15.4). On chest radiography, adenocarcinomas manifest as a solitary pulmonary nodule or mass that can have well-marginated, lobulated, irregular, or spiculated margins. Peripheral adenocarcinomas may directly invade the pleura and grow circumferentially around the lung, mimicking diffuse malignant mesothelioma, metastatic adenocarcinoma of nonlung primary, or malignant thymoma. On computed tomography (CT), adenocarcinomas often have air bronchograms (Fig. 15.5).
Adenocarcinoma of the lung can be classified as (i) preinvasive, (ii) minimally invasive, (iii) invasive, or (iv) a variant of invasive. Preinvasive lesions include atypical adenomatous hyperplasia (AAH), adenocarcinoma in situ (AIS, formerly bronchioloalveolar carcinoma or BAC), nonmucinous, mucinous, and mixed mucinous–nonmucinous adenocarcinoma. AIS is less than or equal to 3 cm with pure lepidic growth. The disease-specific survival is 100% if the lesion is completely resected. Most cases of AIS are nonmucinous. Lepidic growth refers to cuboidal or columnar cells lining the walls of distal airspaces. The pulmonary interstitium serves as scaffolding for tumor growth. Neoplastic cells can detach from the primary tumor and attach to alveolar septa elsewhere in the lung, resulting in multifocal spread of tumor (Figs. 15.6 and 15.7). The cells can produce abundant mucus, giving rise to “bronchorrhea,” the expectoration of large amounts of mucus. Minimally invasive adenocarcinoma (MIA) is a ≤3-cm lepidic-predominant tumor with less than or equal to 5 mm invasion. It is usually nonmucinous but can also be mucinous or mixed mucinous–nonmucinous. Patients should have near 100% disease-specific survival if completely resected. Invasive adenocarcinomas can be lepidic-predominant (LPA, formerly nonmucinous BAC pattern, with >5 mm invasion), acinar-predominant, papillary-predominant, or solid-predominant with mucin production. Variants of invasive adenocarcinoma include invasive mucinous adenocarcinoma (formerly mucinous BAC), colloid, fetal (low and high grade), and enteric.
Table 15.1 COMMON EXTRATHORACIC SITES FOR METASTASES OF LUNG CANCER
“LABB”
Liver
Adrenal
Bone
Brain
Table 15.2 CLINICAL AND RADIOLOGIC FEATURES OF THE FOUR HISTOLOGIC TYPES OF LUNG CANCER
Non–small-cell carcinoma
Adenocarcinoma
Most common type
Weak association with cigarette smoking
Usually peripheral in location
Most common type to have air bronchograms
Adenocarcinoma in situ (formerly bronchioloalveolar carcinoma) is a subtype
Squamous cell carcinoma
Second most common type
Strong association with cigarette smoking
Usually central in location
Most common type to cavitate
Large-cell carcinoma
Least common type
Usually >3 cm in size
Usually in lung periphery
Small-cell carcinoma
Strong association with cigarette smoking
Usually central in location
Often presents with bulky mediastinal adenopathy
Worst prognosis of all types
As one can imagine from the spectrum of different histologies described above, the radiologic patterns of adenocarcinoma are protean (6). Invasive adenocarcinoma is usually a well-circumscribed peripheral solitary nodule or mass (8) (Fig. 15.8) but may also be part solid and occasionally a ground-glass nodule (GGN). A lobar pattern of ground-glass opacity (GGO) may occur. Bubble-like or cystic lucencies in Stage IA adenocarcinoma correlate with well-differentiated tumors and slow growth (Fig. 15.9). Actual cavitation is uncommon, although the so-called “pseudocavitation” is a well-known feature. For Stage IA adenocarcinoma that presents as a part-solid nodule, an extensive ground-glass component correlates with noninvasive growth and suggests a favorable prognosis. A common appearance of MIA is a nodule with a central solid component and peripheral GGO, the so-called “fried egg” sign (Fig. 15.10). Histologically, the ground-glass component typically corresponds to a lepidic pattern and the solid component to invasive patterns. Intratumoral air bronchograms, which are commonly seen, usually indicate a well-differentiated tumor. Invasive mucinous adenocarcinoma, formerly called mucinous BAC, characteristically presents as a range of nodules to lobar replacement by a spectrum of patterns including GGO, mixed GGO/solid foci, or consolidation, but intraalveolar mucus may make the CT appearance solid or nearly solid. The lepidic pattern of growth can look like airspace disease on chest radiography, an appearance similar to that of pneumonia (Fig. 15.11). The mucoid component may appear as homogeneous consolidation with soft tissue attenuation that is lower than that of muscle. After administration of an intravenous iodinated contrast agent, vessels are well shown traversing these regions (CT angiogram sign). AIS can be indolent, multifocal, grow slowly over many months or years, and should always be considered when serial chest radiographs or CT scans show chronic alveolar lung disease (Figs. 15.12 and 15.13).
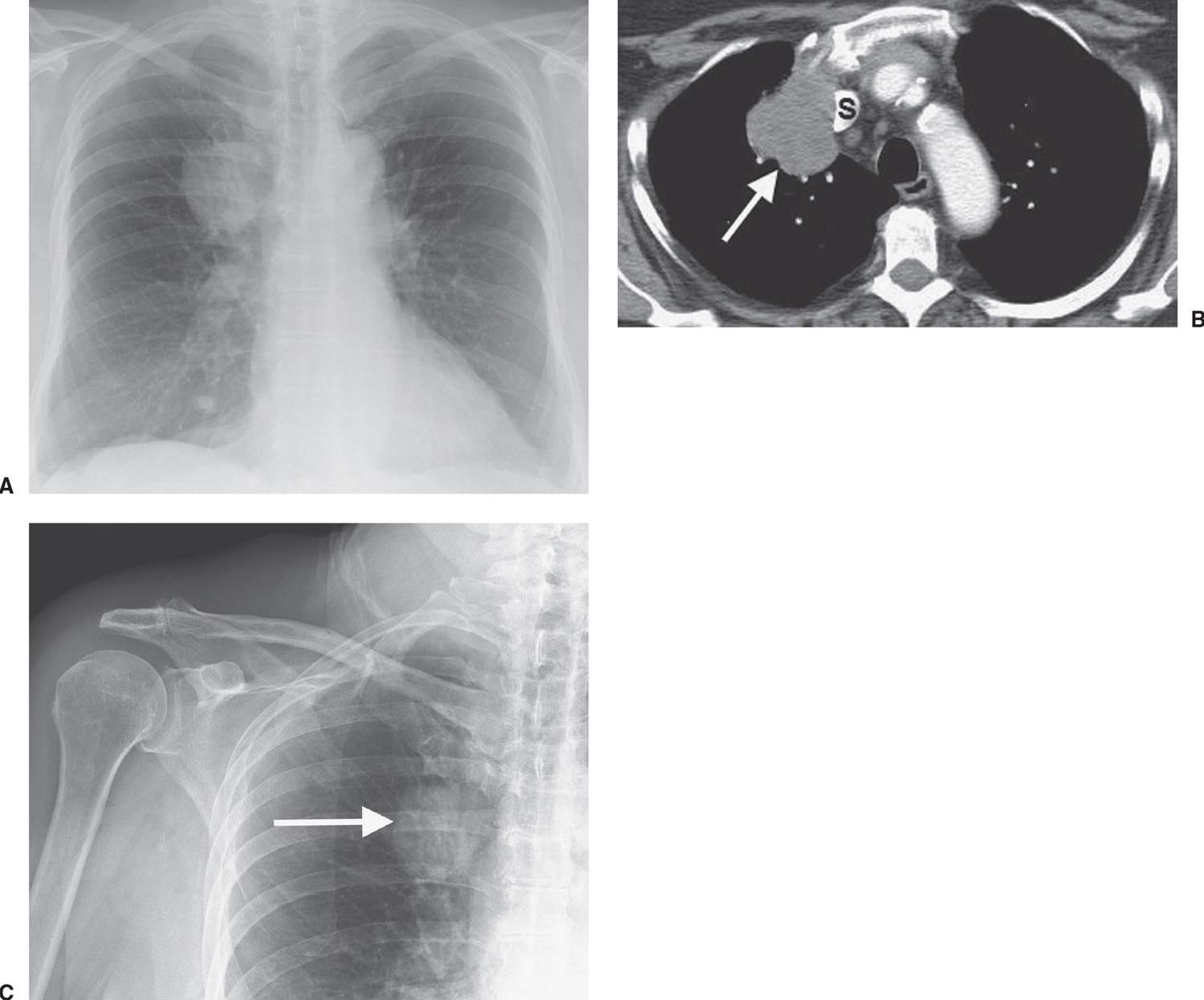
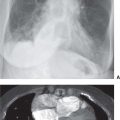
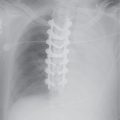
FIG. 15.2 • Adenocarcinoma. A: Posteroanterior (PA) chest radiograph of a 75-year-old woman shows a mass in the right upper lobe abutting the mediastinum. B: CT shows the mass (arrow) compressing the superior vena cava (S). C: The mass (arrow) is seen on a shoulder radiograph obtained 3 months earlier. Incidental lung cancers can be detected on cervical spine and shoulder radiographs, and review of these studies should include a look at the visualized lungs.
Squamous cell carcinoma
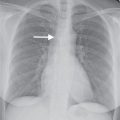
Squamous cell carcinoma is the second most common type of lung cancer, and it is strongly associated with cigarette smoking. It is the most common type to cavitate (Fig. 15.14) and to be associated with hypercalcemia. Microscopically, squamous cell carcinoma is characterized by the presence of intercellular bridges, individual cell keratinization, and formation of keratin pearls. These tumors are most commonly central in location (within the main, lobar, or segmental bronchi), although approximately 25% are peripheral (Fig. 15.15). The typical radiologic manifestations of central squamous cell carcinomas are postobstructive pneumonia and atelectasis because of the total or partial bronchial obstruction produced by these central tumors (Fig. 15.16). The central tumor mass, adjacent to a displaced fissure from obstructive atelectasis, gives rise to the classic radiographic Golden S sign, sometimes referred to as the reverse S sign (see Fig. 2.12).
Peripheral squamous cell carcinoma is the most common type of lung cancer to cause the Pancoast syndrome. In 1924, Henry Pancoast first described a clinical syndrome diagnostic of an apical lung tumor (9). This syndrome is characterized by pain or atrophy of muscles of the ipsilateral upper extremity, caused by involvement of the lower brachial plexus, and Horner syndrome, which results from involvement of the sympathetic chain and the stellate ganglion. Pancoast tumors can manifest as apical masses or asymmetric apical pleural thickening and can be associated with bone destruction (Figs. 15.17 and 15.18) and soft tissue invasion. Magnetic resonance imaging (MRI) is superior to CT in determining whether there is tumor involvement of the chest wall, brachial plexus, subclavian artery, vertebral bodies, and spinal canal.
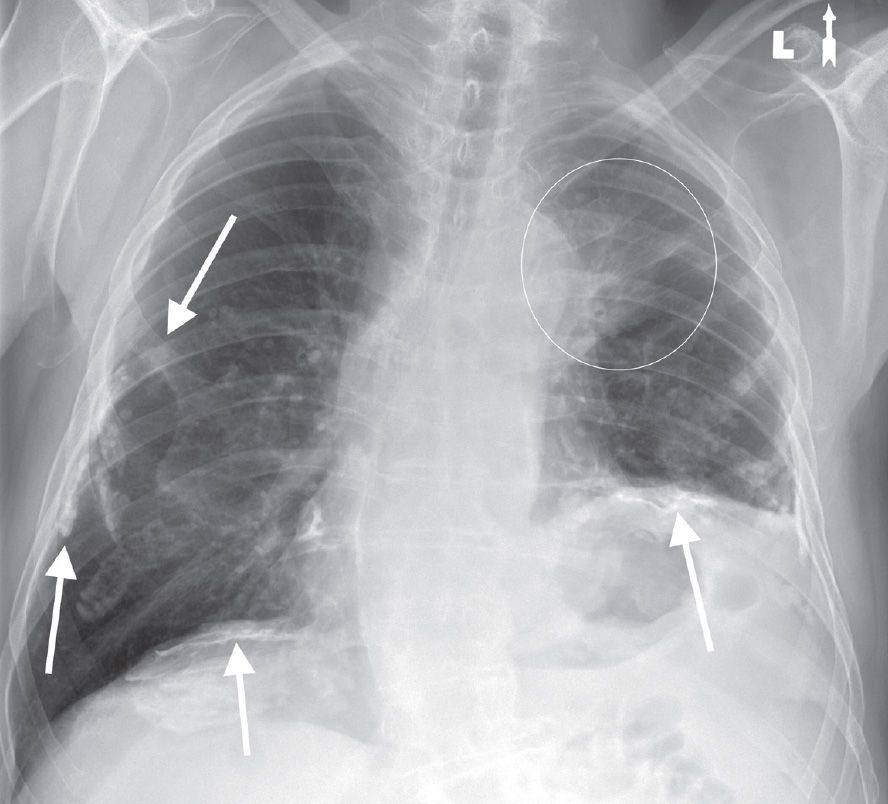
FIG. 15.3 • Adenocarcinoma. PA chest radiograph of a 73-year-old woman with hoarseness and shortness of breath shows calcified pleural plaques (arrows) and a poorly defined mass in the left upper lobe (circle). The pleural plaques are related to previous asbestos exposure. The hoarseness and elevation (paralysis) of the left hemidiaphragm are related to tumor involvement of the left recurrent laryngeal nerve and left phrenic nerve, respectively, in the aortopulmonary window.
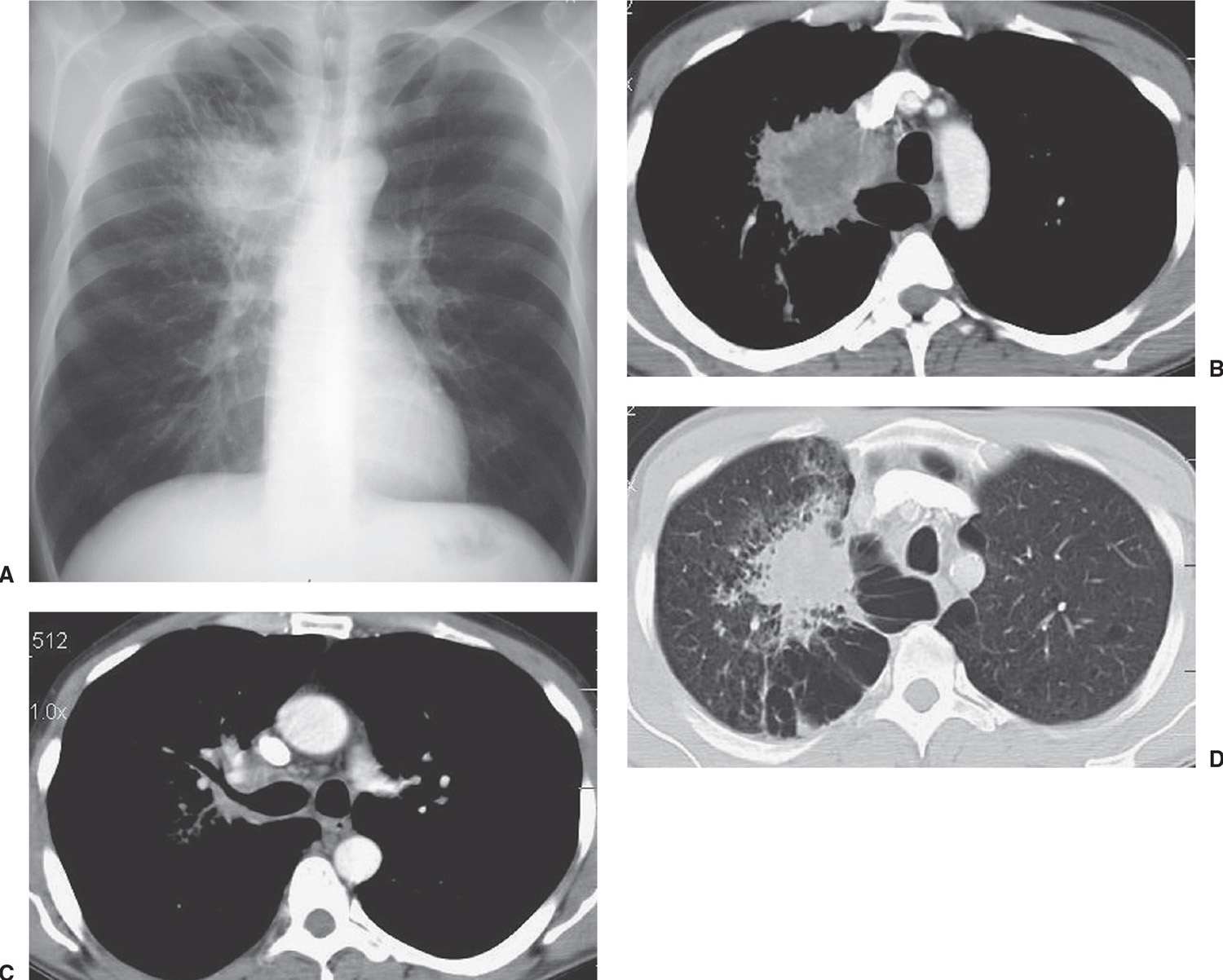
FIG. 15.4 • Adenocarcinoma. A: PA chest radiograph of a 48-year-old man shows an irregular mass in the right upper lobe abutting the mediastinum. B: CT shows the mass extending into the mediastinum. The center of the mass is of low attenuation, secondary to tumor necrosis. C: CT at a level inferior to (B) shows tumor along the posterior wall of the right upper lobe bronchus. D: CT with lung windowing shows the spiculated mass and a background of paraseptal and centrilobular emphysema.
Large-cell carcinoma
These tumors are the least common type of lung cancer. They grow rapidly, metastasize early, and are strongly associated with cigarette smoking. The histologic diagnosis is one of exclusion, given only to lung cancers that lack features of squamous, glandular, or small-cell differentiation. Large-cell carcinomas are appropriately named: they are usually bulky tumors greater than 3 cm in diameter. They are typically located in the lung periphery, but central lesions are not uncommon (Fig. 15.19). The typical radiologic appearance of these tumors is a large peripheral lung mass (10).
Small-cell carcinoma
Small-cell carcinoma is a rapidly growing neoplasm characterized by early and widespread metastases and by a strong association with cigarette smoking. Histologically, small-cell carcinoma is characterized by small, uniform, oval cells with scant cytoplasm. Extensive crushing artifact is frequently seen in bronchial biopsy specimens, reflecting the tumor’s scant tumor stroma and lack of desmoplastic reaction. Small-cell carcinoma has been classified as a “neuroendocrine neoplasm” of the lung, and it is the most common cell type to cause a clinical hormone syndrome by secreting ectopic hormones. The majority of these tumors are located centrally within lobar and mainstem bronchi. They have extensive necrosis and hemorrhage, invade adjacent structures and lymph nodes, and disseminate along lymphatic routes.
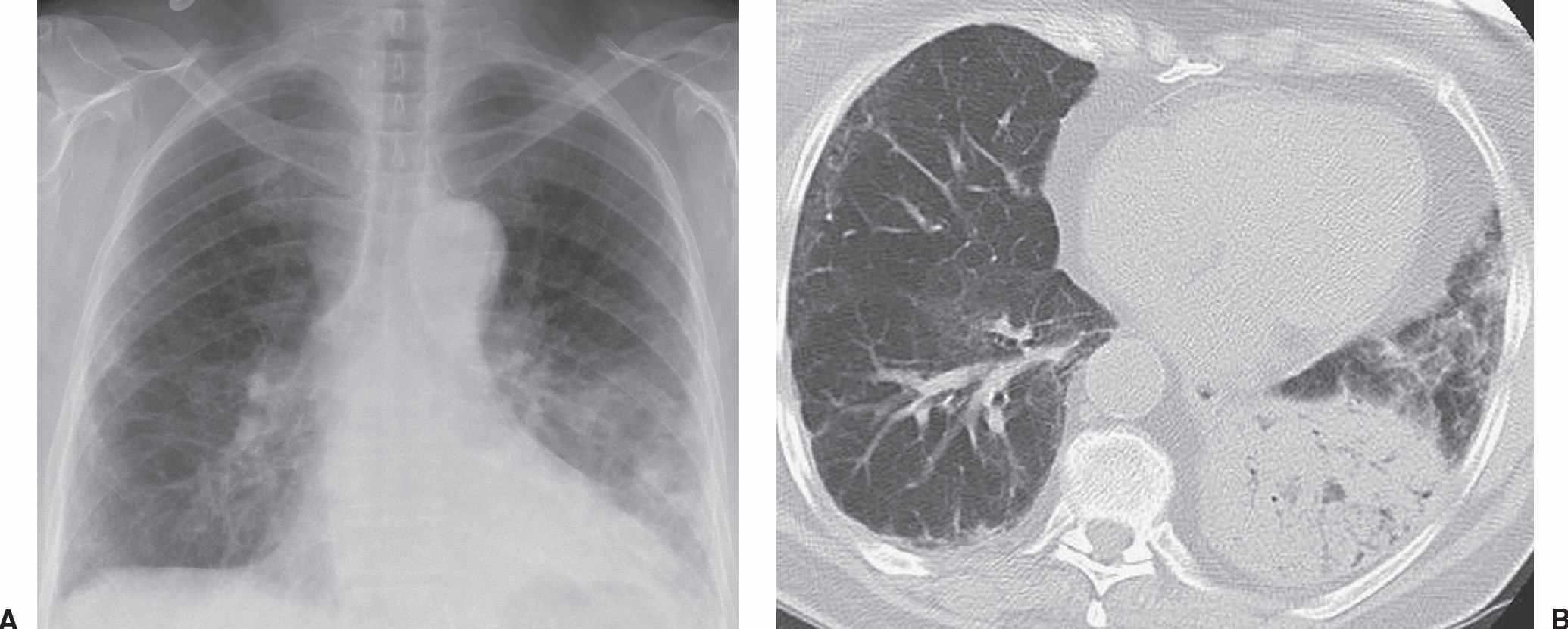
FIG. 15.5 • Adenocarcinoma with adenocarcinoma in situ component. A: PA chest radiograph of a 73-year-old woman with chronic cough and symptoms of pneumonia for 3 months shows airspace disease in the left lower lung. B: CT shows numerous air bronchograms within the left lower lobe airspace opacity. The patient was treated with antibiotics for presumed lobar pneumonia before the diagnosis of cancer was made. Adenocarcinoma, particularly AIS, should be considered when chest radiographs show chronic airspace disease.
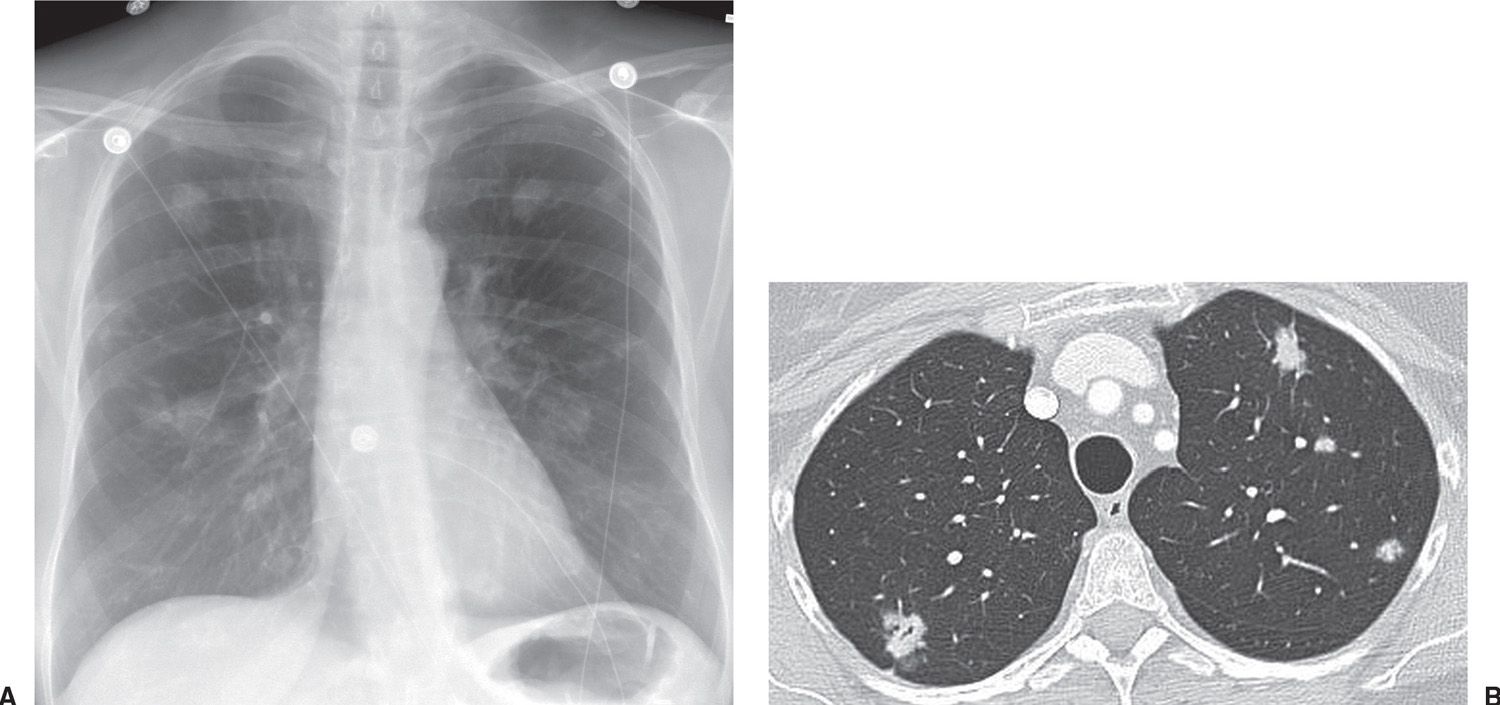
FIG. 15.6 • Multifocal adenocarcinoma in situ. A: PA chest radiograph shows bilateral pulmonary nodules. B: CT shows small bilateral mixed-attenuation nodules. An air bronchiologram is seen in the right upper lobe nodule.
The chest radiograph usually shows a hilar or perihilar mass associated with mediastinal widening; this can be caused by the primary tumor, metastases to hilar/mediastinal lymph nodes, or a combination of both. The primary tumor may not be evident, and nodal enlargement may be the dominant abnormality. Rarely, small-cell carcinoma may manifest as a solitary pulmonary nodule or mass (Fig. 15.20). CT usually shows extensive mediastinal lymph node involvement, with soft tissue “infiltration” of the mediastinum similar to that seen with lymphoma (Figs. 15.21 to 15.23). Small-cell carcinoma is the most common primary lung cancer to cause superior vena cava obstruction, secondary to extrinsic vascular compression by the tumor, endoluminal thrombosis, or invasion (11). Surgical resection is considered in selected patients with small-cell carcinoma only when the tumor manifests as a solitary pulmonary nodule in the absence of metastases. Most patients have disseminated disease at presentation and undergo chemotherapy and radiation therapy (Fig. 15.24). The response to this treatment is usually dramatic, and the mass can disappear in a relatively short period of time, but most patients still die with rapidly recurrent small-cell carcinoma (12).
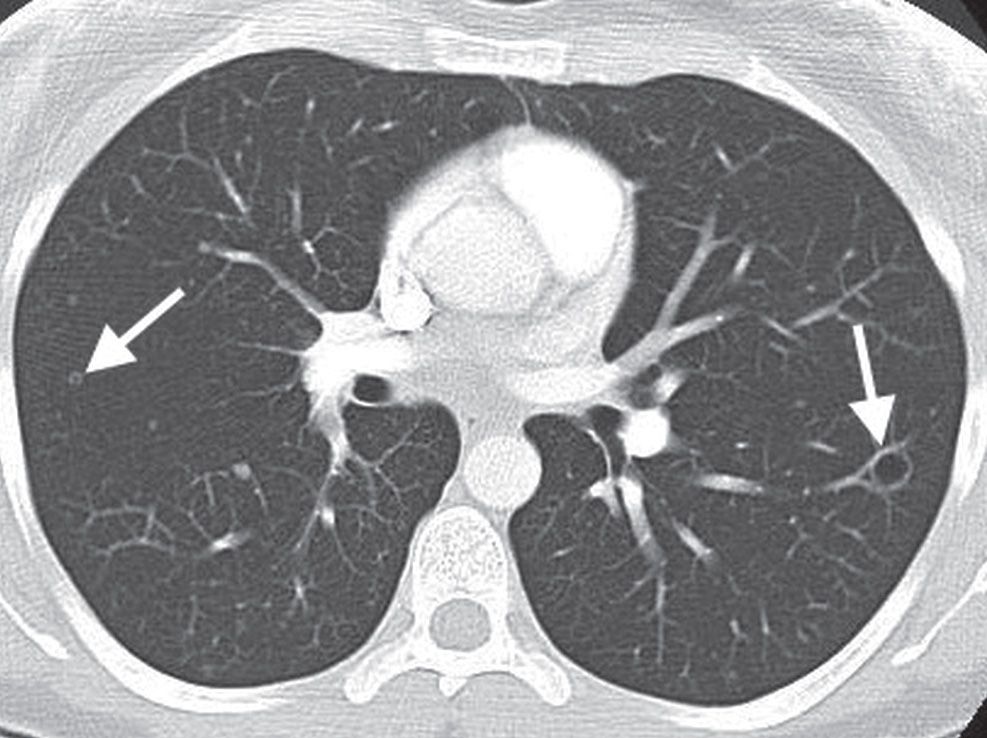
FIG. 15.7 • Multifocal adenocarcinoma in situ. CT shows small, bilateral nodules, some of which appear cystic (arrows). This cystic appearance of multifocal AIS (formerly bronchioloalveolar carcinoma) has been called the “cheerios” sign.
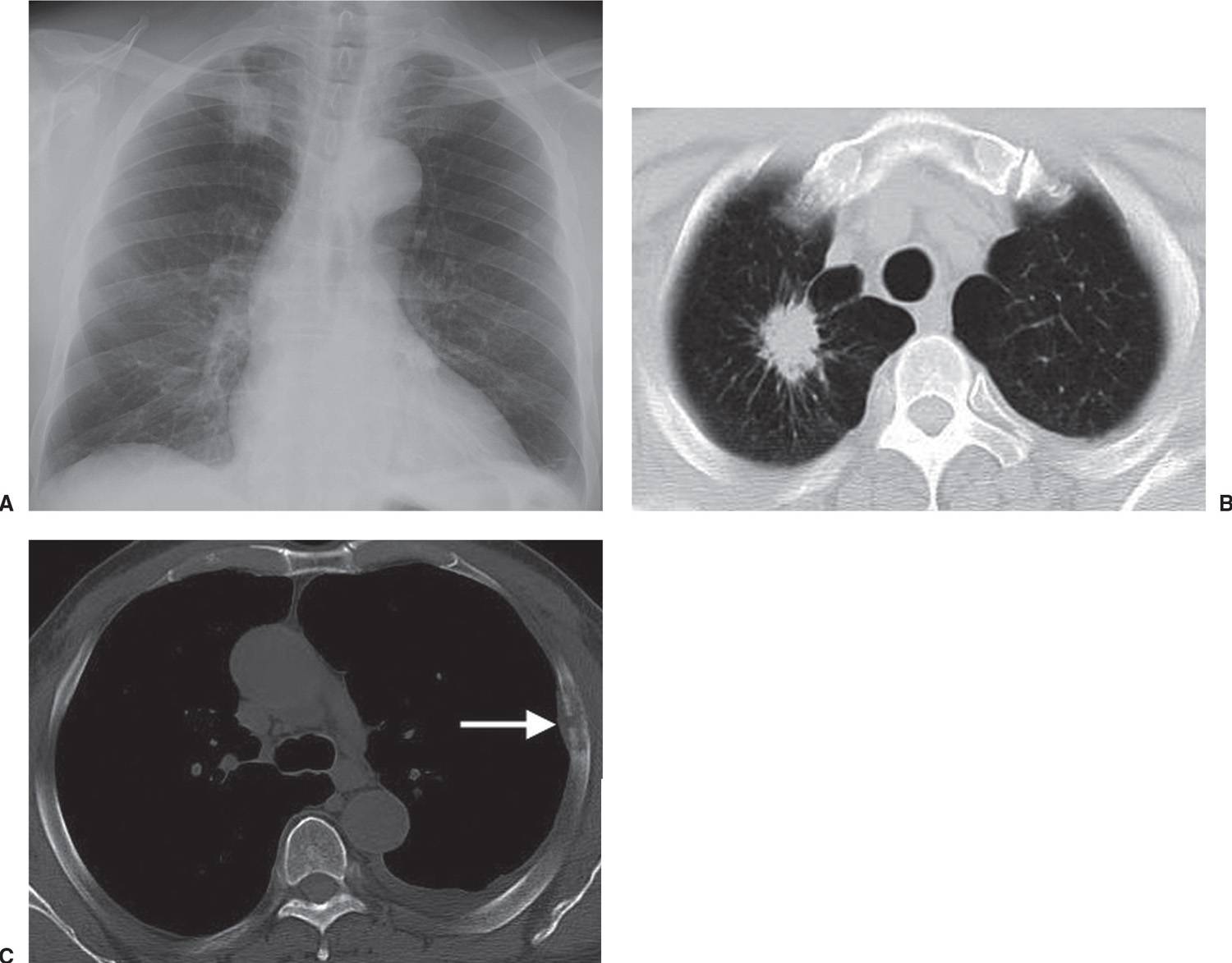
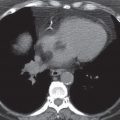
FIG. 15.8 • Invasive adenocarcinoma. A: PA chest radiograph of a 72-year-old man with a 53 pack-year history of cigarette smoking shows a mass in the right upper lobe. B: CT shows a spiculated mass and a background of centrilobular emphysema. C: CT with bone windowing shows a destructive lesion of the left fifth lateral rib (arrow). Bone metastasis makes this a Stage IV tumor.
Staging of Lung Cancer
Staging differs between small-cell and non–small-cell lung cancer (NSCLC). Small-cell carcinoma is generally considered inoperable, except in rare cases of small, localized tumors. It is staged as limited or extensive, depending on whether the disease is confined to a single radiation port (limited) (Fig. 15.25) or not (extensive) (Fig. 15.26). Patients with limited disease receive radiation therapy and chemotherapy, whereas patients with extensive disease receive only chemotherapy.
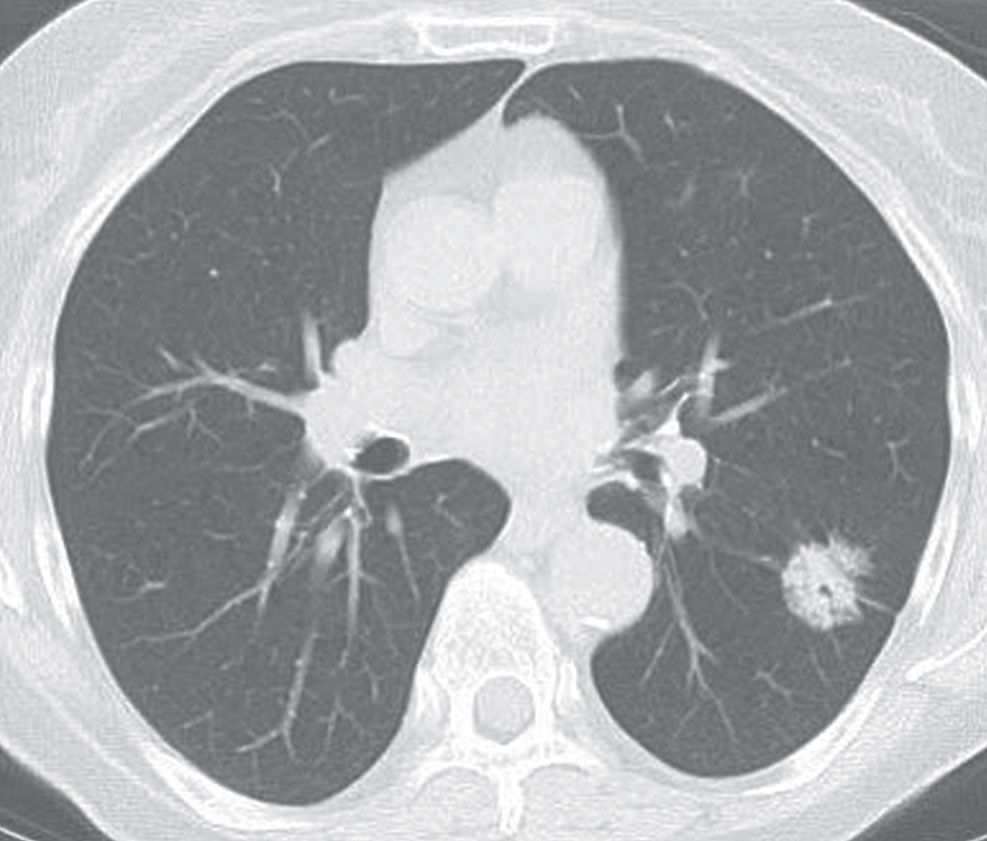
FIG. 15.9 • Adenocarcinoma in situ. CT shows a 2.6-cm mixed-attenuation nodule with central lucencies. Air bronchograms/bronchiolograms within a nodule are a characteristic feature of AIS.
The primary goal of staging NSCLC is to determine resectability. The 7th edition of the TNM (tumor-node-metastases) International System for Staging Lung Cancer was developed by the International Association for the Study of Lung Cancer (IASLC) and approved by the American Joint Committee on Cancer (AJCC) and the International Union Against Cancer (UICC) to replace the sixth edition effective January 1, 2010 (13,14). The major change in the seventh edition was the reclassification of malignant pleural effusions and separate tumor nodule(s) (previously called satellite nodules). Other changes included new size cutoffs and new subdivisions of the T1 (into T1a and T1b) (Fig. 15.27), T2 (into T2a and T2b), and M1 (into M1a and M1b) descriptors (Table 15.3). No changes to the N descriptor were made. Stage grouping involves the concept of combining subsets of patients classified according to TNM descriptors into categories or stages, with each having generally similar treatment options and survival expectations (Table 15.4). Twenty to 30% of patients with NSCLC are diagnosed with stage I to stage IIIA disease and, thus, may be amenable to surgical resection. The recent IASLC staging project demonstrated overall 5-year survival of 73% for stage IA, 58% for stage IB, 46% for stage IIA, 36% for stage IIB, 24% for stage IIIA, and 9% for stage IIIB (15).
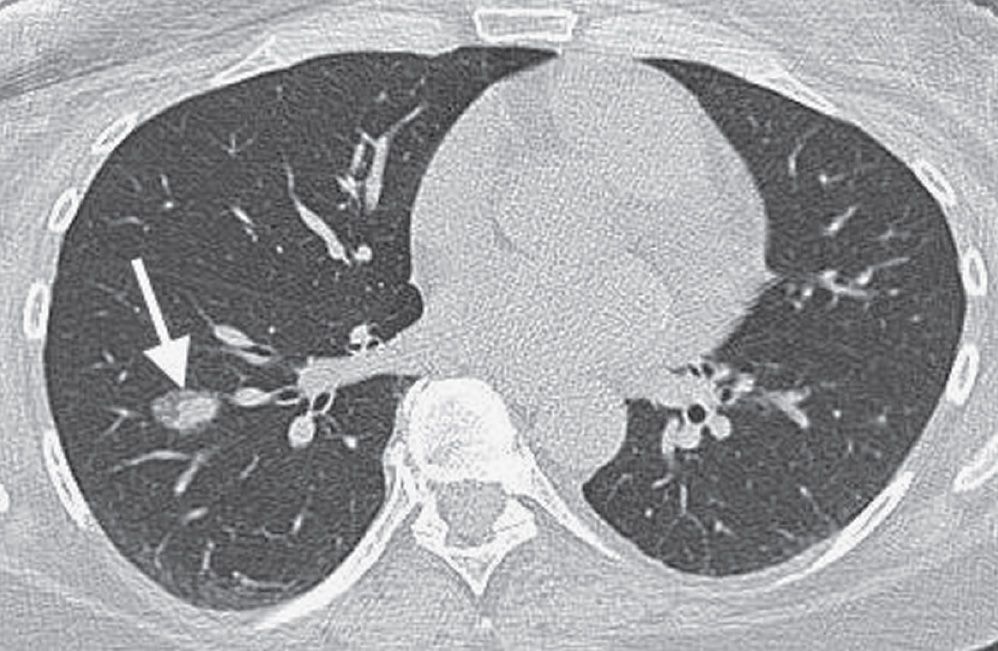
FIG. 15.10 • Minimally invasive adenocarcinoma. CT of a 52-year-old woman with an 11 pack-year history of cigarette smoking shows an incidental right lower lobe nodule (arrow). The nodule has a central dense component and a peripheral ground-glass component, giving rise to the “fried egg” appearance that is characteristic of MIA. The patient underwent right lower lobectomy for a stage IA (T1N0M0) cancer.
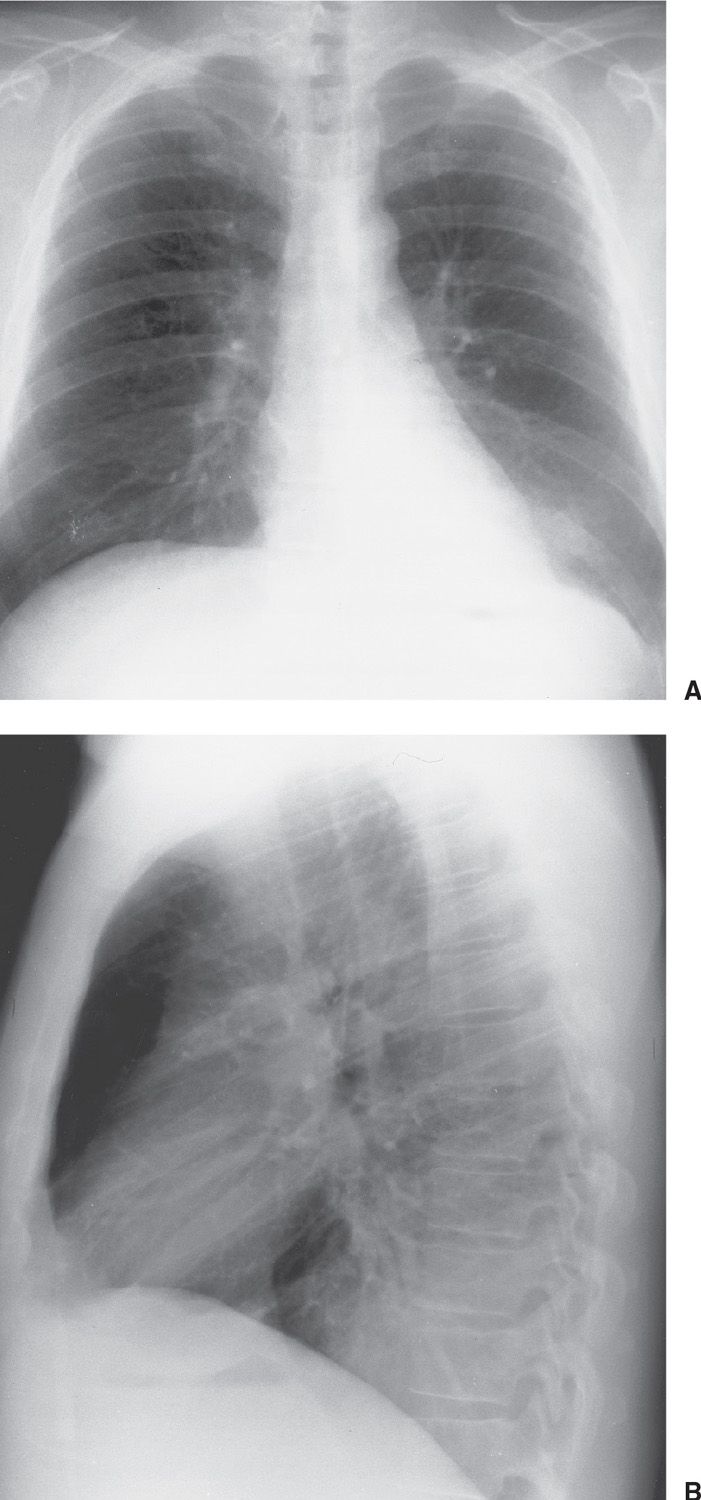
FIG. 15.11 • Invasive mucinous adenocarcinoma (formerly mucinous bronchioloalveolar carcinoma). A: PA chest radiograph shows focal airspace disease in the left lower lobe, obscuring the medial left hemidiaphragm. B: Lateral view shows increased opacification over the lower thoracic spine (the so-called “spine sign”). The appearance is similar to that of left lower lobe pneumonia.
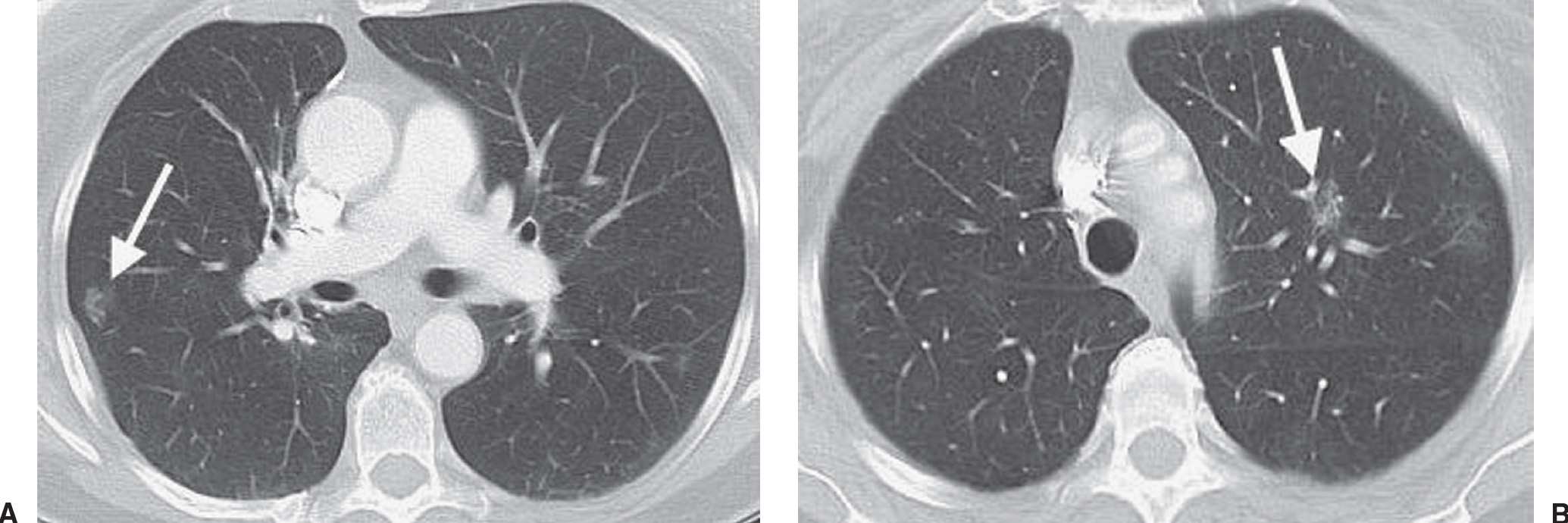
FIG. 15.12 • Bilateral adenocarcinoma in situ. A: CT of a 71-year-old woman with a 30 pack-year history of cigarette smoking and resection of AIS in the right upper lobe 4 years earlier shows a GGN in the right lower lobe (arrow). B: CT at a level superior to (A) shows a GGN in the left upper lobe (arrow). Both nodules were proven to represent AIS. GGNs are very worrisome for AIS, especially in a patient with a history of this type of cancer.
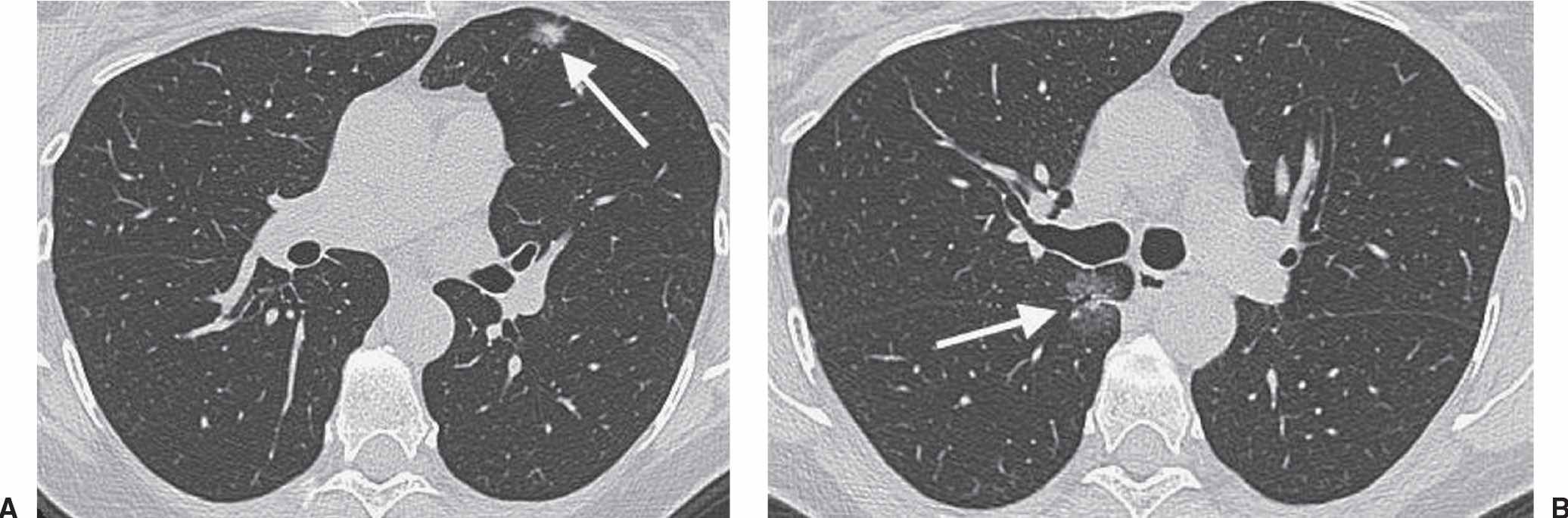
FIG. 15.13 • Recurrent adenocarcinoma in situ. A: CT scan shows a mixed-attenuation nodule in the left upper lobe (arrow). The patient underwent lingulectomy to remove an AIS. B: CT image obtained 2 years later shows a GGN with an air bronchogram in the medial right lung (arrow). Wedge resection of the right upper lobe and superior segment of the right lower lobe confirmed the recurrence of AIS.
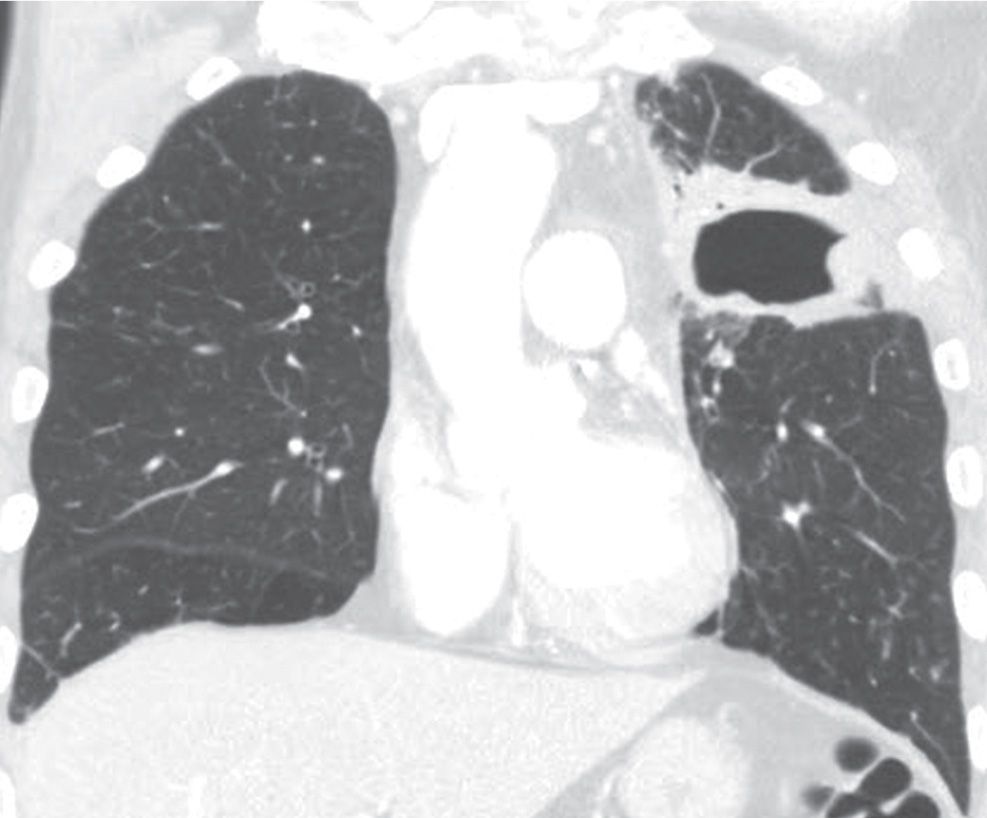
FIG. 15.14 • Squamous cell carcinoma. Coronal CT shows a cavitary mass in the left upper lobe.
Tumor classification is the most complicated component of the TNM system. Tumors that are classified as anything other than T4 are potentially resectable. T4 tumors invade the mediastinum, heart, great vessels, trachea, esophagus, recurrent laryngeal nerve, vertebral body, or carina; or they are associated with separate tumor nodule(s) located in a different lobe of the ipsilateral lung (Figs. 15.28 and 15.29).
Hilar node involvement is classified as N1. N2 nodes are ipsilateral mediastinal or subcarinal nodes, and N3 nodes are contralateral mediastinal or hilar nodes. N3 nodes also include any ipsilateral or contralateral scalene or supraclavicular lymph nodes (Fig. 15.30).
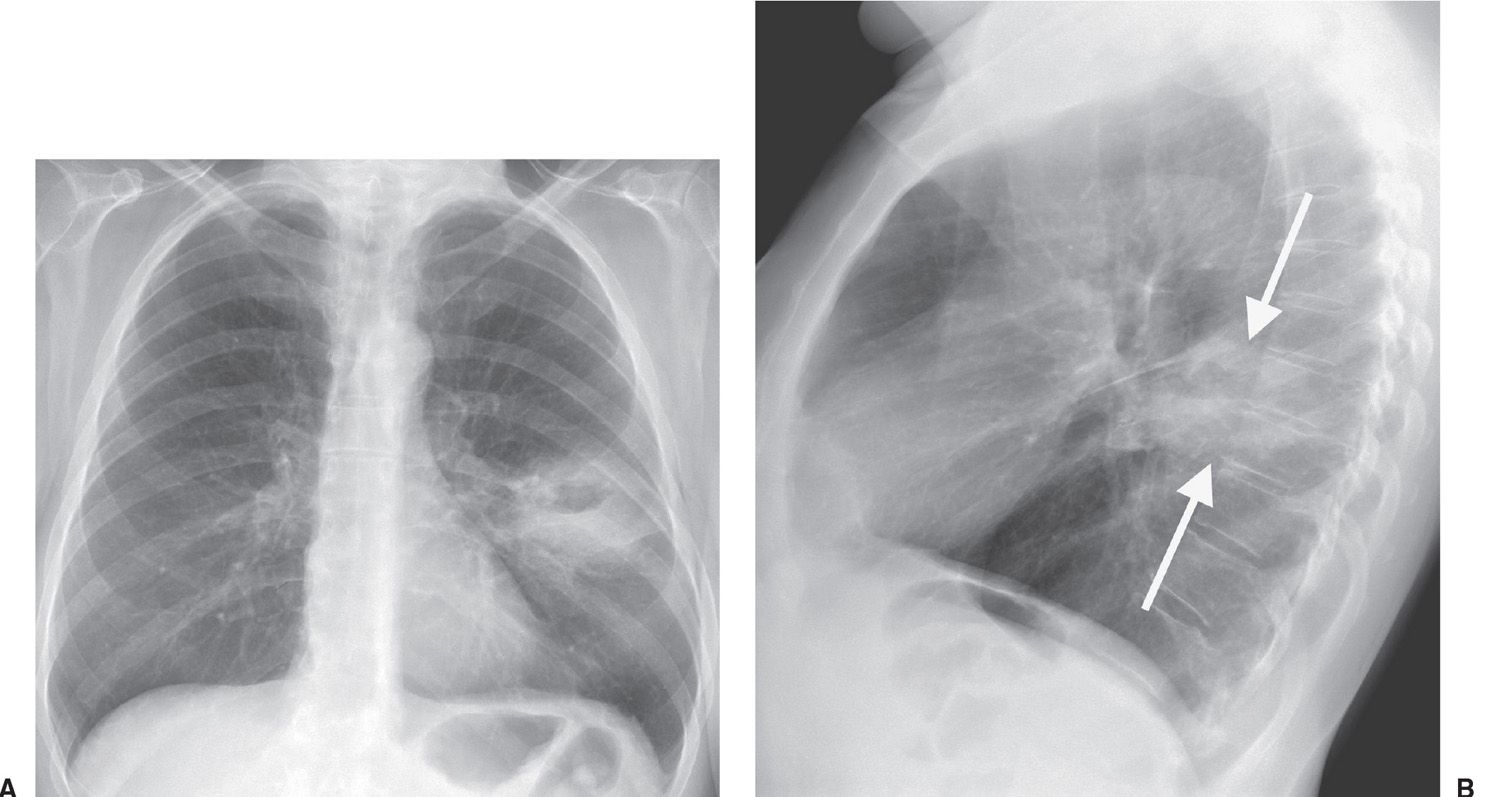
FIG. 15.15 • Squamous cell carcinoma. A: PA chest radiograph of a 62-year-old woman with left chest pain shows an ill-defined mass with central lucency in the left midlung. B: Lateral view confirms that this mass is in the superior segment of the left lower lobe (arrows).
The distant metastases classification is simple. M0 indicates no distant metastases, M1a is malignant pleural effusion, pericardial effusion, pleural nodules, or metastatic nodules in the contralateral lung, and M1b are distant metastases (Figs. 15.31 and 15.32). Most pleural effusions associated with lung cancer are malignant, but cytologic proof of malignancy cannot always be obtained. In these cases, the effusion should be excluded as a staging element.
The staging system is complicated and difficult to remember unless one routinely evaluates and stages lung cancer. Patients with T1N0M0 tumors (Stage IA) have a significantly better outcome than patients in the other subsets (16,17) (Fig. 15.33). These patients have a tumor that is 3 cm or less in diameter, surrounded by lung or visceral pleura, without bronchoscopic evidence of invasion more proximal than the lobar bronchus, and without nodal involvement or metastases. In other words, these are patients with a solitary pulmonary nodule and no spread of tumor. Stage IB tumors also have no nodal or distal metastases, but the primary tumor is either larger than 3 cm in diameter, involves the main bronchus, invades the visceral pleura, or is associated with atelectasis or obstructive pneumonitis. Sixty-one percent of patients with clinical stage IA disease and 38% of those with clinical stage IB tumors are expected to survive more than 5 years after treatment. IA and IB subsets have no evidence of lymph node or other metastases and therefore have the best prognosis.
Stage IIA, IIB, and IIIA tumors are potentially resectable, although the prognosis after treatment is poor, especially with IIIA tumors (Fig. 15.34). Some surgeons opt not to resect IIIA tumors for this reason. IIIB staging involves either T4 tumors or N3 nodes, making such tumors unresectable. IIIB tumors are confined to the lung, however, which is an important consideration for radiotherapy. Stage IV tumors are defined by an M1 classification and are therefore unresectable and not confined to the lung. If treated, systemic therapy is required.
CT is routinely used for staging lung cancer prior to surgical resection. Patients with bulky N3 nodes are clearly not surgical candidates, and those patients without evidence of nodal involvement are considered surgical candidates (in the absence of T4 or M1 disease). However, CT is not perfect in detecting nodal involvement. In general, nodes greater than 1 cm in short-axis diameter are considered positive or suspicious, but many of these cases will turn out to be false-positive findings. In addition, nodes that are smaller than 1 cm or not visibly enlarged on CT can be positive histologically. Nodes can be sampled percutaneously, transbronchially, or via transcervical mediastinoscopy (requiring general anesthesia). The Chamberlain procedure involves an anterior thoracotomy, usually with removal of the second anterior rib to allow sampling of lymph nodes in the anterior mediastinum, the aorticopulmonary window, and the hilum. Other limitations of CT include the inability to determine mediastinal or chest wall invasion with certainty. MRI plays a role in evaluating these cases, as well as in evaluating for the presence of brachial plexus invasion.
Whole-body positron emission tomography (PET) imaging with [18]-fluoro-2-deoxy-D-glucose (FDG) has become an integral part of staging NSCLC since it was approved for this purpose in 1998 (18). Integrated PET/CT scanners allow for the acquisition of coregistered, spatially matched functional and morphologic data. Because of its superior sensitivity, PET/CT has replaced PET as the standard for staging NSCLC in North America. PET improves the detection of nodal and distant metastases and frequently alters patient management (19) (Fig. 15.35). PET is sufficiently sensitive that a patient with negative mediastinal PET results may proceed directly to surgical resection of the primary tumor without a staging mediastinoscopy (20). Sensitivity of PET for AIS is usually very low. PET is commonly used for staging and follow-up of invasive adenocarcinoma, and for lesions of 7 mm or larger, the standard uptake value (SUV) for adenocarcinoma of the lung tends to be lower than for other histologic types of lung cancer and correlates inversely with survival (6). PET/CT also plays a major role in the assessment of treatment response and has significant prognostic value.
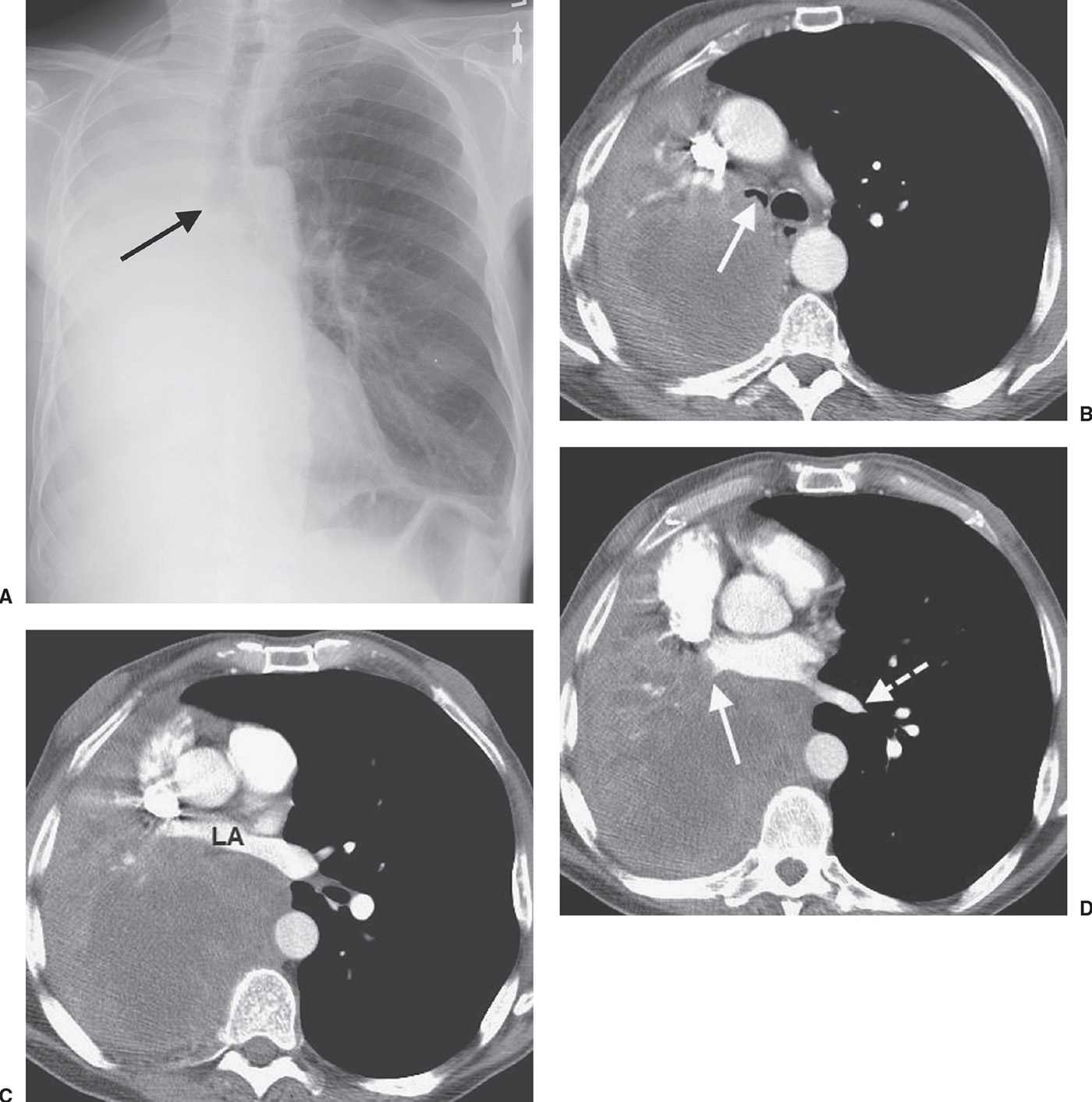
FIG. 15.16 • Squamous cell carcinoma. A: PA chest radiograph of a 63-year-old man with hemoptysis, cough, and dyspnea on exertion shows collapse of the right lung. The right main bronchus appears to be cut off (arrow). The right hemithorax is opaque and the mediastinum is shifted to the right. B: CT shows a mass that almost completely obliterates the lumen of the right main bronchus (arrow). The large, low-attenuation mass extends out into the right lung. C: CT at a level inferior to (B) shows anterior compression of the left atrium (LA) by the mass. D: CT at a level inferior to (C) shows tumor obliteration of the right inferior pulmonary vein (solid arrow). Note the normal left inferior pulmonary vein (dashed arrow). The appearance of a central tumor with postobstructive pneumonia and atelectasis secondary to total or partial bronchial obstruction is typical of squamous cell carcinoma.
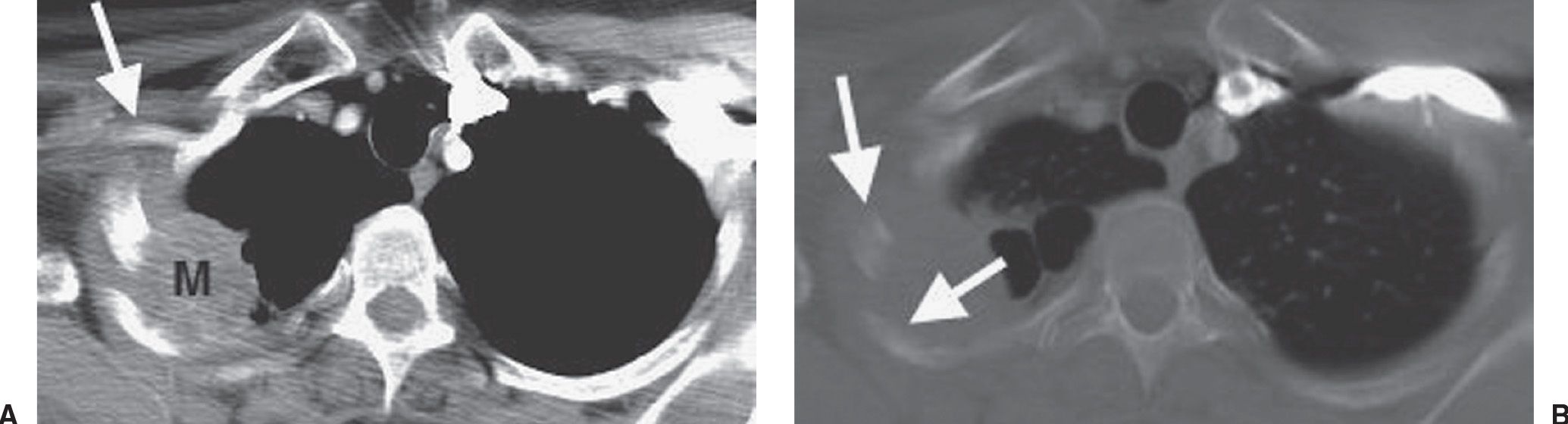
FIG. 15.17 • Pancoast tumor. A: CT of a 54-year-old man with pain in the right suprascapular area radiating down the medial right forearm, a 60 pack-year history of cigarette smoking, and previous exposure to asbestos shows a right apical mass (M) involving the posterior chest wall and rib. The mass is in close proximity to the right axillary artery (arrow), which is suspicious for brachial plexus involvement by tumor. B: CT with bone windowing confirms rib involvement by tumor (arrows). The patient underwent induction chemotherapy and radiation, followed by right upper lobectomy. At surgery, the tumor was found to be invading the 2nd through the 5th ribs.
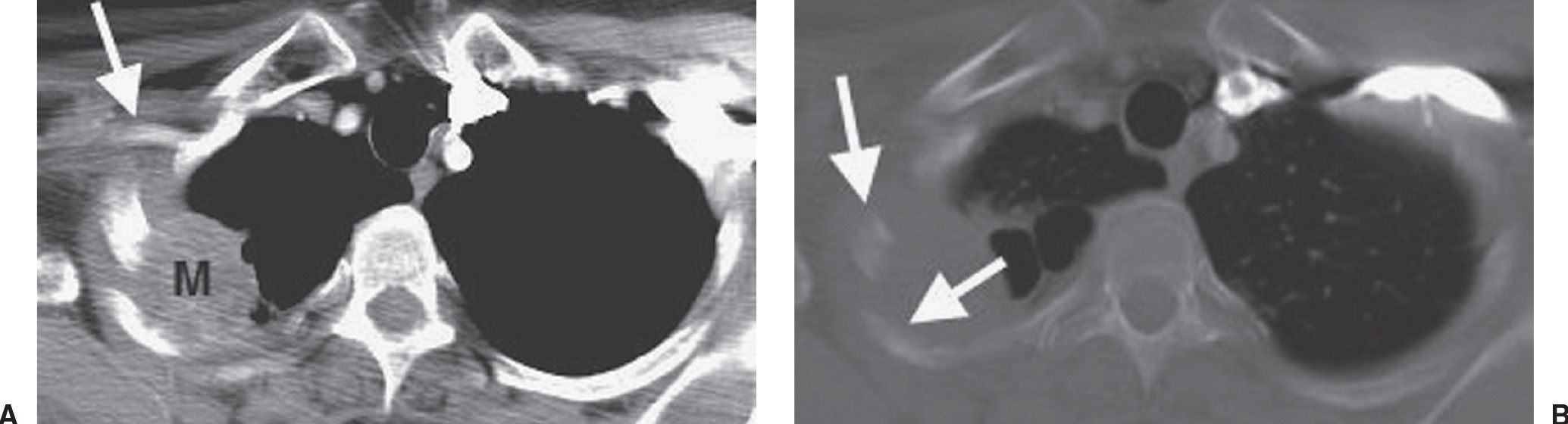
FIG. 15.18 • Pancoast tumor. Coronal CT shows a mass in the right apex (solid arrow) and erosion of the adjacent rib (dashed arrow).
Lung Cancer Screening
The National Lung Screening Trial (NLST) was a randomized control trial with over 50,000 patients aged 55 to 74 years at the time of randomization who had a tobacco use history of at least 30 pack-years and, if former smokers, had quit within the past 15 years (21). The report of the NLST showed a 20% reduction in lung cancer mortality with CT screening of the chest. The U.S. Preventive Services Task Force (USPSTF) recommended annual screening for lung cancer with low-dose CT in adults aged 55 to 80 years who have a 30 pack-year smoking history and currently smoke or have quit within the past 15 years. Screening should be discontinued once a person has not smoked for 15 years or develops a health problem that substantially limits life expectancy or the ability or willingness to have curative lung surgery (Screening for lung cancer: U.S. Preventive Services Task Force Recommendation Statement. Annals of Internal Medicine. Published as online-first version at www.annals.org on December 31, 2013. The final version may differ in small ways). Other organizations that recommend screening for lung cancer using LDCT include The Society of Thoracic Radiology, the American College of Chest Physicians, the American Society of Clinical Oncology, the American Thoracic Society, the American Association for Thoracic Surgery, The American Cancer Society, and the National Comprehensive Cancer Network (22).
In the NLST, 96% of lung nodules found were ultimately determined to be false positives and 39% of patients had at least one positive result during the study (23). The National Comprehensive Cancer Network published guidelines for lung cancer screening, including protocols for follow-up, workup, and invasive testing of positive findings, similar to those recommended by the Fleischner Society (24,25).
POSTPNEUMONECTOMY COMPLICATIONS
In the United States, the most common indication for pneumonectomy is non–small-cell carcinoma of the lung. Most pneumonectomies performed for lung cancer follow an interpleural plane of resection (meaning the parietal pleura is left intact). If there is extension of tumor into the pleural space or parietal pleura, or in the case of malignant mesothelioma, an extrapleural pneumonectomy is generally performed. In this case, the plane of resection is between the parietal pleura and the endothoracic fascia (26).
After pneumonectomy, pleural fluid accumulates in the pneumonectomy space, replacing the normal immediate postoperative air that is resorbed at a variable rate. It is not uncommon for multiple air–fluid levels to be present within the early pneumonectomy space, representing loculation of fluid, and this finding on chest radiography does not necessarily suggest a complication. Most of the air is resorbed by 2 weeks after pneumonectomy; residual air may persist for months however, or, in a small population of patients, it may never be completely resorbed. Eventually, the pneumonectomy space will contract, with ipsilateral shift of the mediastinum and elevation of the diaphragm, and the space will fill with fluid and some degree of solid fibrothorax. Shift of the mediastinum away from the operated side indicates a buildup of air or fluid within the pneumonectomy space. Mediastinal displacement away from the operative side suggests one of five diagnoses, depending on the length of time after surgery (Table 15.5). If the air–fluid level has not continued to rise after surgery, the cause of the contralateral mediastinal shift is likely a bronchial stump air leak. If the air–fluid level has continued to rise, the shift can be a result of hemothorax, chylothorax, or empyema, with or without a bronchopleural fistula. A drop in the air–fluid level indicates that fluid is draining through a chest tube, by thoracentesis, through a dehiscence of the incision, through an opening in the bronchial stump (Fig. 15.36), or through a rent in the diaphragm (26). After the postoperative period, shift of the mediastinum away from the operative side is also suspicious for recurrent tumor (Fig. 15.37), which can be recognized on CT as a soft tissue mass at the site of surgical ligation and soft tissue deposits studding the periphery of the pneumonectomy space. Recurrence can also be seen in the remaining lung (Fig. 15.38). PET/CT scanning is routinely used to evaluate for recurrence.
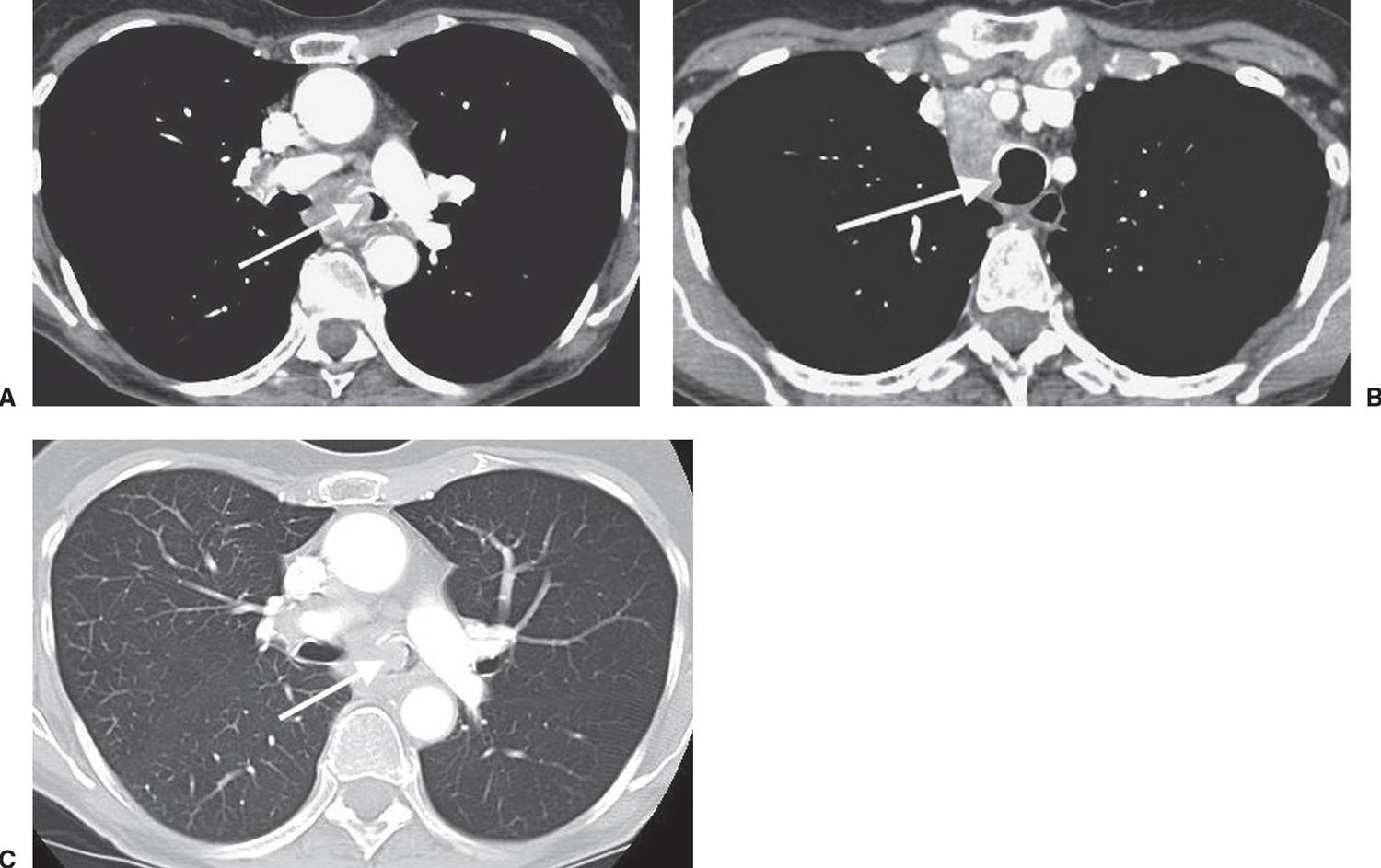
FIG. 15.19 • Large-cell carcinoma. A: CT of an 80-year-old woman with dyspnea, wheezing, cough, fatigue, 12-lb weight loss, and a history of cigarette smoking shows a mass partially obstructing the left main bronchus (arrow). B: CT at a level superior to (A) shows compression of the adjacent trachea (arrow). C: CT with lung windowing shows hyperlucency related to air trapping on the left secondary to a ball-valve effect from central obstructing tumor (arrow).
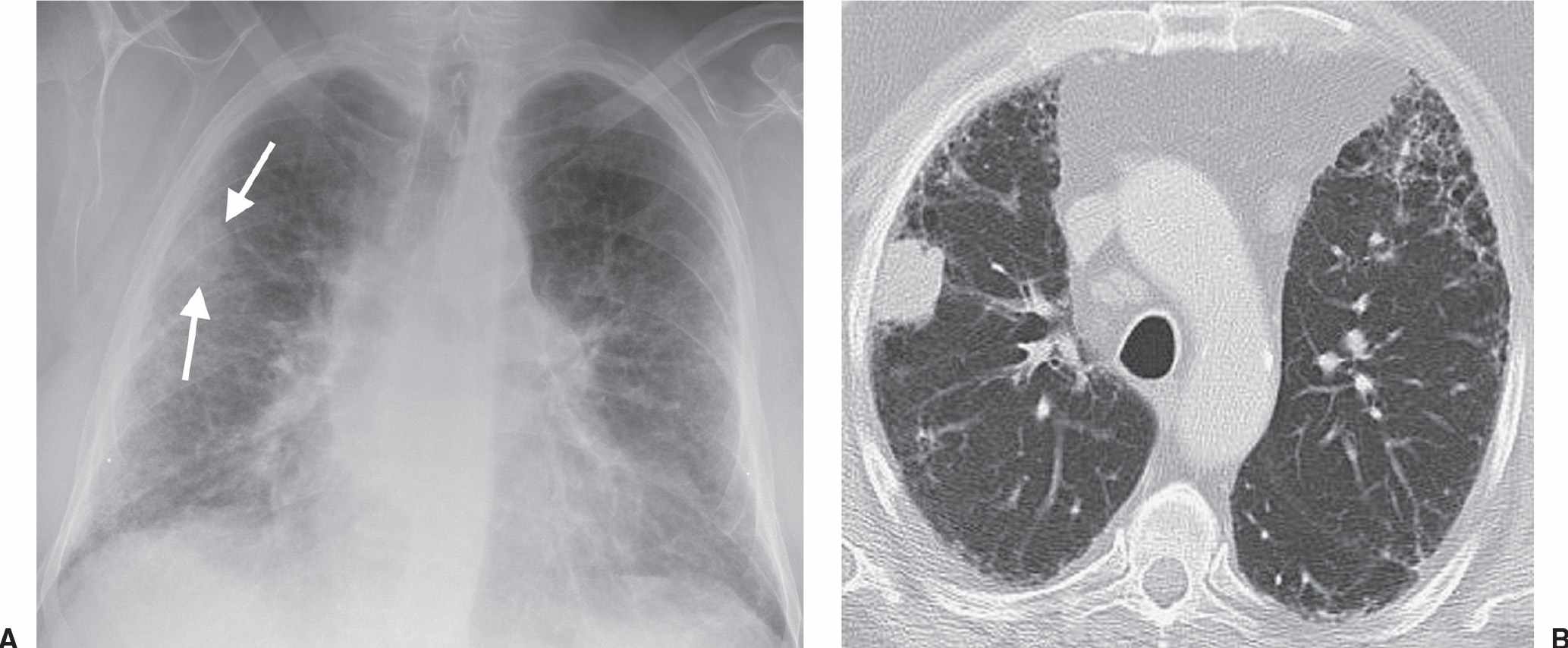
FIG. 15.20 • Small-cell carcinoma. A: PA chest radiograph of a patient with pulmonary fibrosis, obtained as part of a workup for lung transplantation, shows a nodule (arrows) in the right lung. B: CT shows a subpleural nodule in the right upper lobe. Note the bilateral, subpleural, reticular, interstitial lung disease. Wedge resection confirmed a stage IB cancer. This is a known but uncommon appearance of small-cell lung cancer, which usually presents with extensive lymph node involvement and widespread metastases.
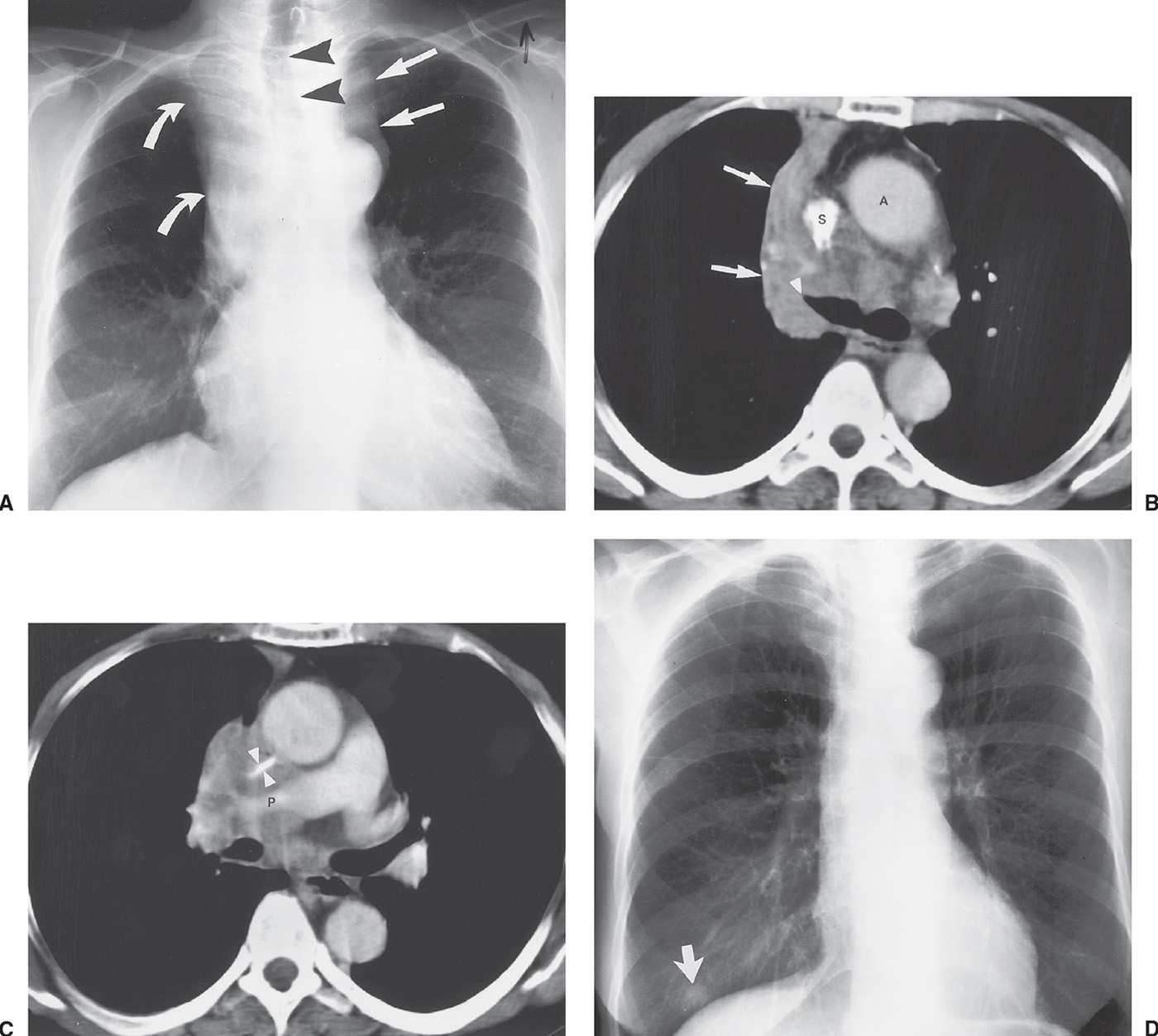
FIG. 15.21 • Small-cell carcinoma. A: PA chest radiograph of a 56-year-old woman with weight loss and malaise shows widening of the left mediastinal contour (straight arrows), right hilar convexity, and collapse of the right upper lobe, with elevation of the minor fissure (curved arrows). The trachea is displaced to the right (arrowheads). B: CT shows abrupt tapering of the right upper lobe bronchus (arrowhead) and collapse of the right upper lobe against the mediastinum (arrows). The tumor infiltrates the mediastinum posterior to the ascending aorta (A) and superior vena cava (S). C: CT at a level inferior to (B) shows encasement and slitlike compression of the superior vena cava (arrowheads) and right pulmonary artery (P) by tumor. D: PA chest radiograph obtained 4 months later, after chemotherapy and radiation therapy, shows marked regression of tumor. A nipple shadow is incidentally projected over the right lung base (arrow).
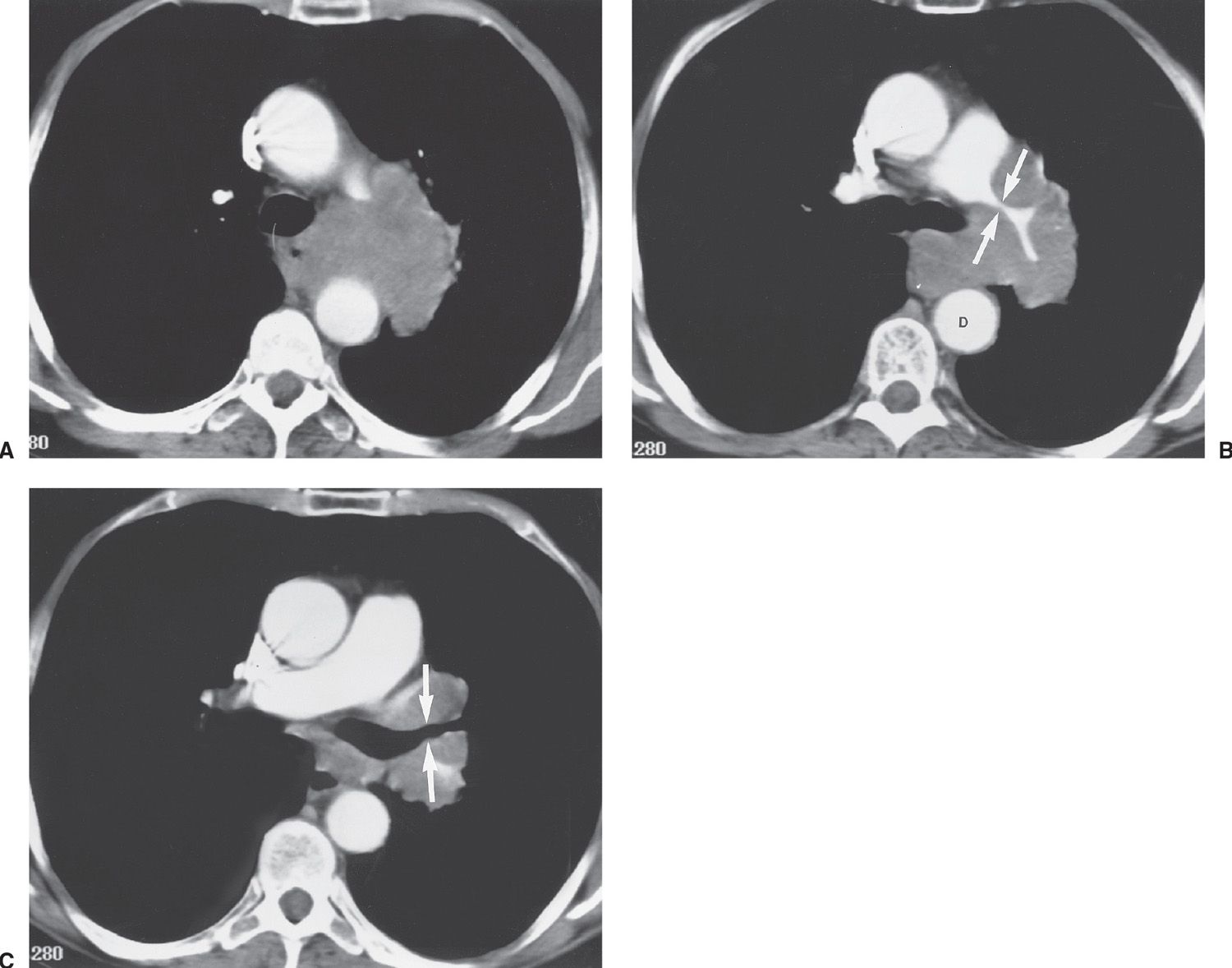
FIG. 15.22 • Small-cell carcinoma. A: CT of a 57-year-old woman with hoarseness shows tumor infiltrating the aortopulmonary window and invading the left recurrent laryngeal nerve. B: CT at a level inferior to (A) shows encasement of the left pulmonary artery (arrows) by tumor and extension of tumor posterior to the carina, obliterating the fat plane adjacent to the descending aorta (D). C: CT at a level inferior to (B) shows encasement of the left upper lobe bronchus by tumor (arrows).
The surgical mortality of pneumonectomy is approximately 6%, with the major causes of death being pneumonia, respiratory failure, pulmonary embolism, myocardial infarction, bronchopleural fistula, and empyema (27,28). The incidence of empyema is 2% to 5%, often with associated bronchopleural fistula (29). In the first postoperative week, empyema is caused by intraoperative soilage or preoperative pleural infection. Delayed onset of empyema is often associated with bronchopleural or esophagopleural fistula. New air within the pneumonectomy space, in a previously opacified hemithorax, with contralateral shift of the mediastinum, is suggestive of empyema or bronchopleural fistula and bronchial stump leak (Fig. 15.39).
A rare complication of right pneumonectomy is obstruction of the left main bronchus, a result of extreme rightward shift and counterclockwise rotation of the mediastinum, causing compression of the left bronchus between the aorta and the left pulmonary artery. This complication is termed the “right pneumonectomy syndrome,” and it can occur between 1 and 37 years after surgery (30). The diagnosis is suggested on chest radiography by marked mediastinal shift to the right and inversion of the left diaphragm, caused by the trapping of air from a narrowing of the left bronchus (26). There can also be recurrent left lower lobe pneumonia resulting from airway obstruction.
CARCINOID AND SALIVARY GLAND TUMORS
The term bronchial adenoma refers to a group of tumors that includes bronchial carcinoid (most common), mucoepidermoid carcinoma, and adenoid cystic carcinoma. This term, however, is not accurate, as adenoma implies a benign tumor, and many of these tumors are not benign. Additionally, adeno- implies glandular elements, which are sometimes lacking in these tumors. Carcinoid tumors have a different cell of origin, and adenoid cystic and mucoepidermoid carcinomas are classified as salivary gland tumors. Because this legacy term exists in the radiology literature and is still used by some clinicians, it is discussed in this chapter.
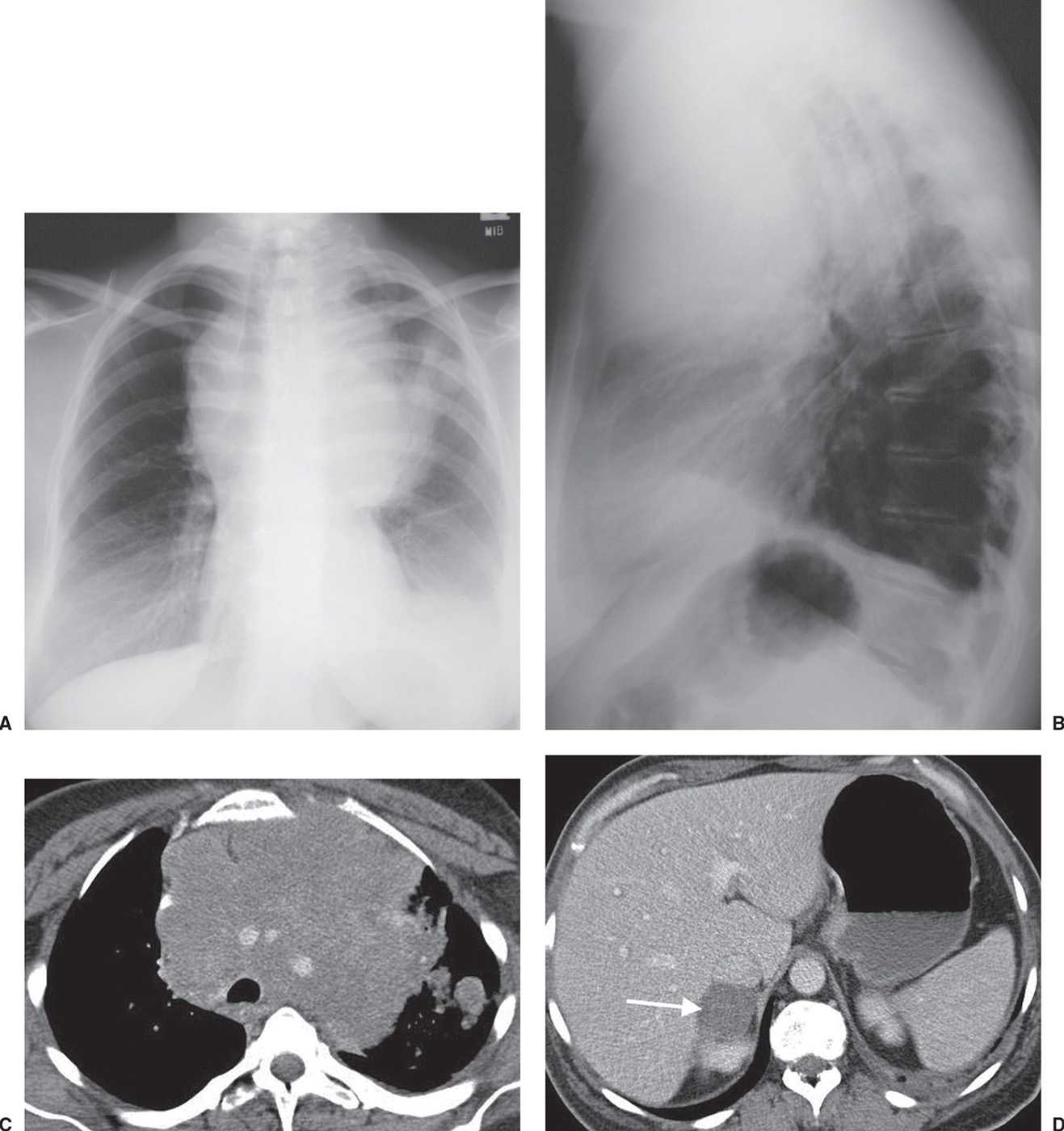
FIG. 15.23 • Small-cell carcinoma. A: PA chest radiograph shows a large “mediastinal” mass associated with rightward shift of the trachea. B: Lateral view shows the mass filling the anterior mediastinum and posterior displacement of the trachea. C: CT shows mediastinal infiltration by tumor that encases and narrows the lumen of enhancing vascular structures. Ill-defined nodules are visible in the left lung. D: CT at a level inferior to (C) shows a right adrenal metastasis (arrow).
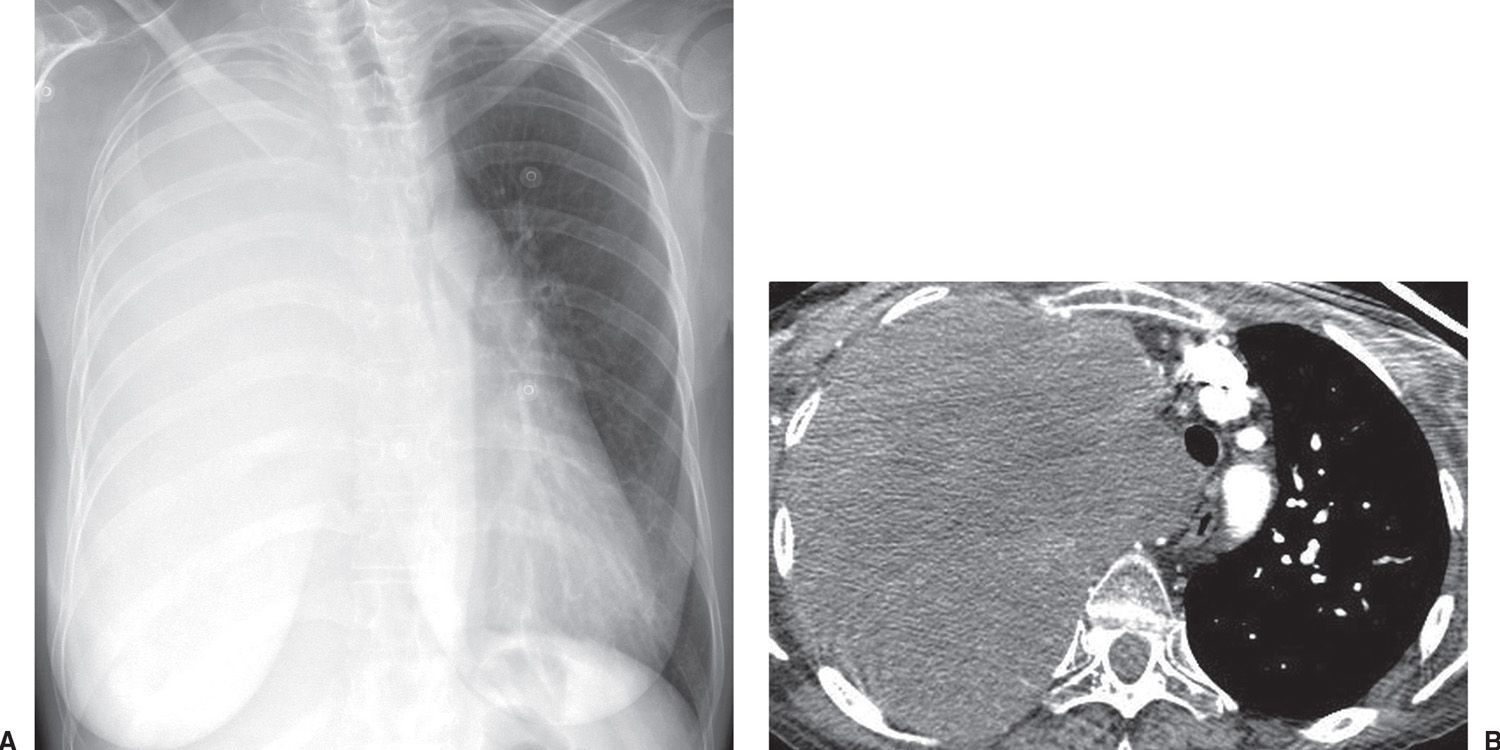
FIG. 15.24 • Small-cell carcinoma. A: PA chest radiograph shows opacification of the right hemithorax and leftward shift of the mediastinum. B: CT shows tumor filling the right hemithorax, invading the mediastinum.
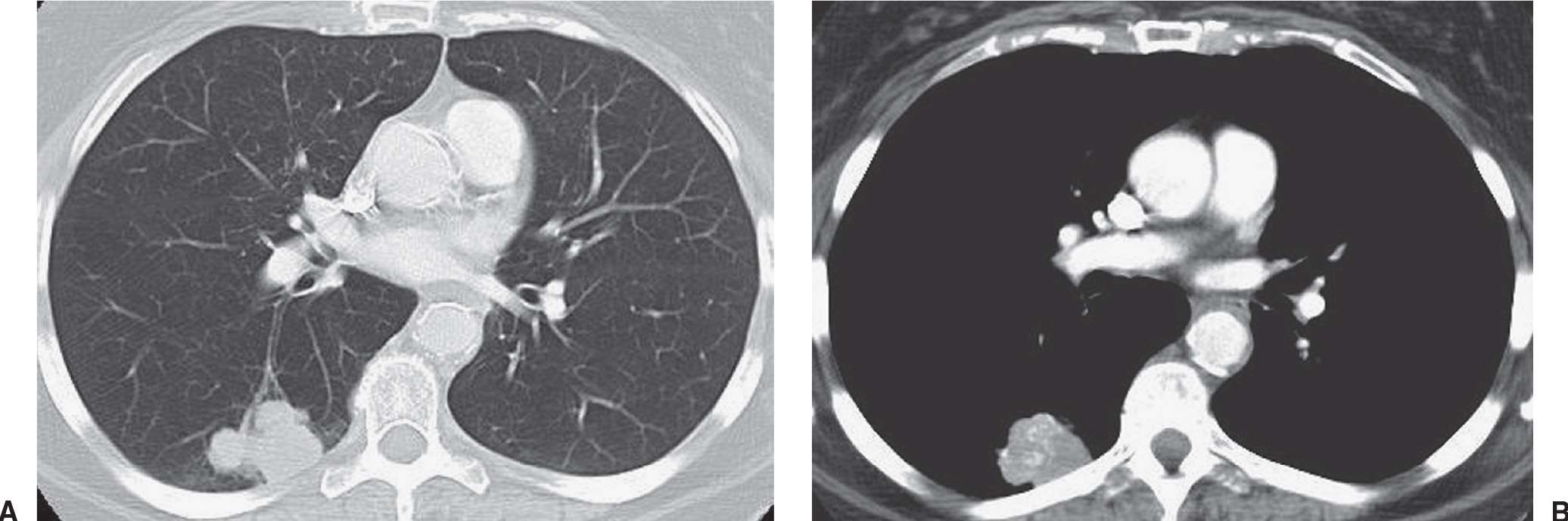
FIG. 15.25 • Small-cell carcinoma, limited stage. A: CT of a 64-year-old woman shows a lobulated mass in the right lower lobe. B: CT with mediastinal windowing shows calcification or contrast enhancement within the mass. Mediastinal lymphadenopathy was present in the right paratracheal area (not shown). CT and PET showed no evidence of extrathoracic tumor. The patient received radiation therapy and chemotherapy.
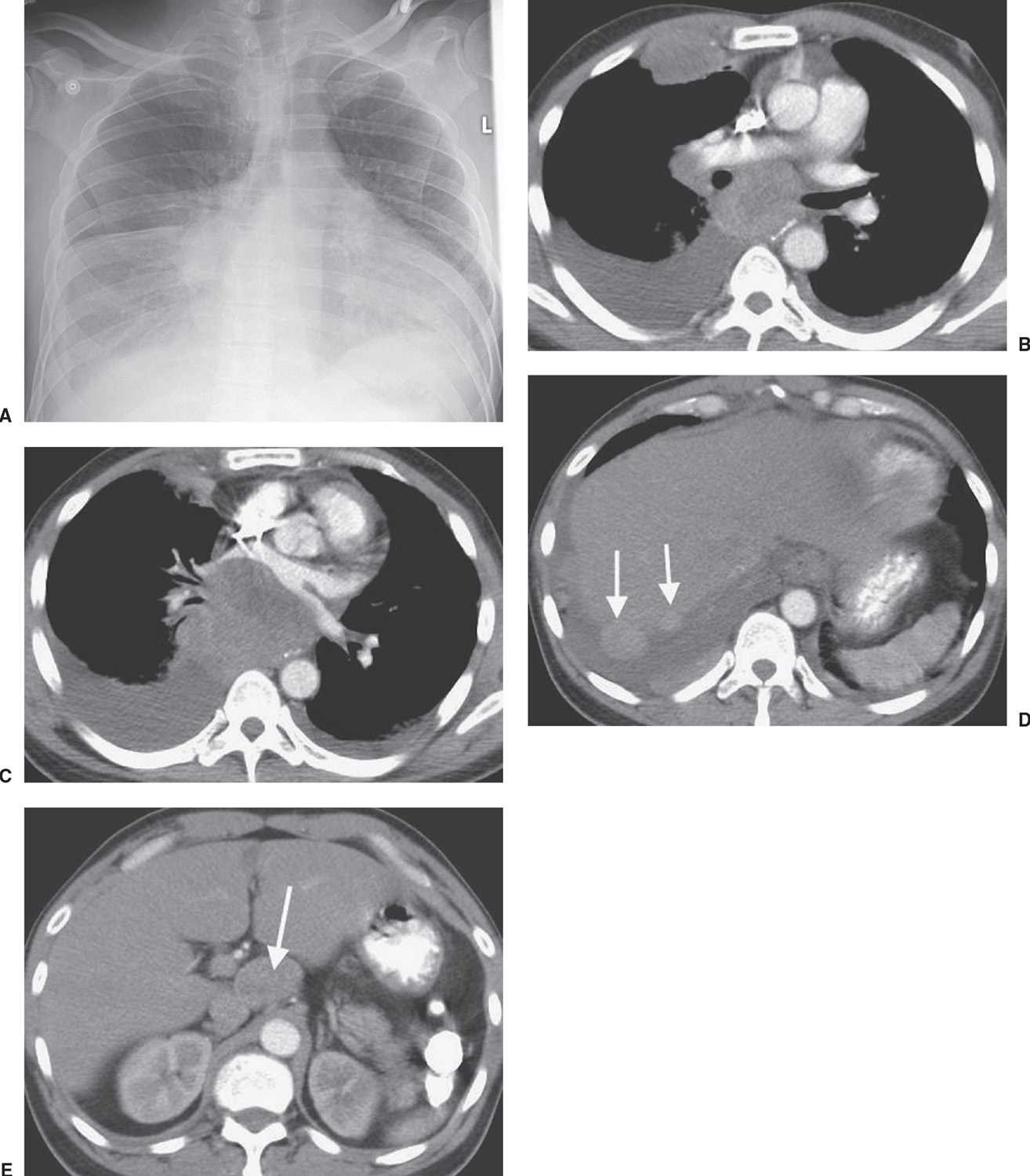
FIG. 15.26 • Small-cell carcinoma, extensive. A: PA chest radiograph of a 47-year-old man with abdominal pain and vomiting shows enlargement of the cardiac silhouette, right pleural effusion, and abnormal opacities in the right paratracheal area, right hilum, and both lung bases. B: CT shows bilateral, pleural effusions, bulky subcarinal lymphadenopathy, and a large, anterior right pleural mass. C: CT at a level inferior to (B) shows anterior displacement of the left atrium by bulky tumor. D: CT at a level inferior to (C) shows numerous pleural tumor deposits (arrows). E: CT of the upper abdomen shows bulky celiac lymphadenopathy (arrow). The patient received chemotherapy.
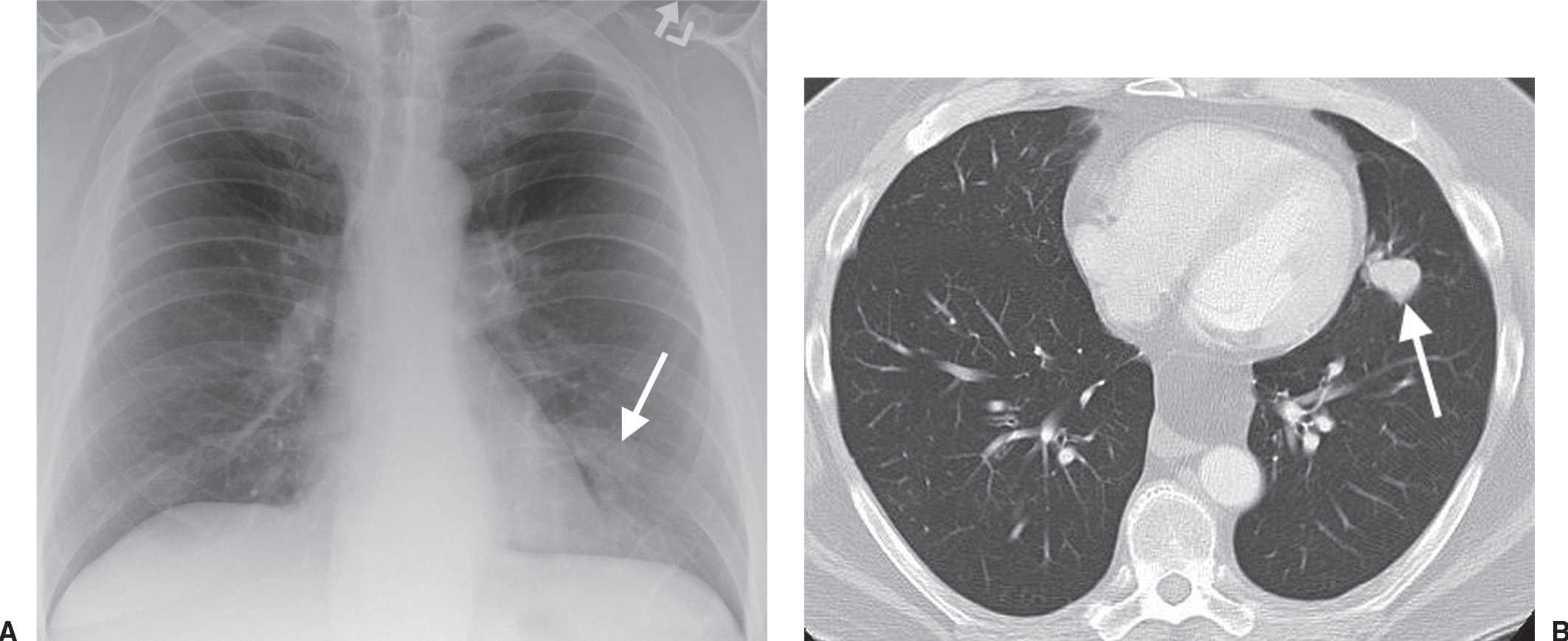
FIG. 15.27 • Stage IA lung cancer. A: PA chest radiograph shows a nodule adjacent to the left heart border (arrow). B: CT shows a 2.5-cm nodule (arrow) in the lingula. The size makes this a T1b tumor. There was no nodal involvement or metastases, making this a Stage IA tumor.
Table 15.3 TNM DESCRIPTORS FOR STAGING LUNG CANCER
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree