LEARNING OBJECTIVES
1. Identify and describe the findings of the following on a chest radiograph or computed tomography (CT):
• Atrial septal defect
• Ventricular septal defect
• Patent ductus arteriosus
2. Identify and describe the findings of the following on a chest radiograph:
• Enlarged left atrium
• Enlarged right ventricle
• Enlarged left ventricle
3. Identify and describe the chest radiographic findings, and state the most common etiologies of each of the following valvular diseases:
• Aortic stenosis
• Aortic insufficiency
• Mitral stenosis
• Mitral insufficiency
• Tricuspid stenosis
• Tricuspid insufficiency
• Pulmonic stenosis
• Pulmonic insufficiency
4. Recognize an enlarged ascending aorta and aortic valve calcification on a chest radiograph, and suggest the diagnosis of aortic stenosis when these findings are present.
5. Recognize an enlarged left atrium, vascular redistribution, and mitral valve calcification on a chest radiograph, and suggest the diagnosis of mitral stenosis when these findings are present.
6. Describe the radiographic appearance of mitral annulus calcification, and name the cardiac diseases associated with mitral annulus calcification.
7. Define the types of cardiomyopathy (dilated, hypertrophic, restrictive), and list the common causes of each.
8. Identify on CT and describe the CT findings and clinical significance of lipomatous hypertrophy of the interatrial septum.
9. Define arrhythmogenic right ventricular dysplasia, describe the role of magnetic resonance imaging (MRI) in its diagnosis, and identify MRI findings that support the diagnosis.
10. Name the most common benign primary cardiac tumors, including myxoma, lipoma, fibroma, and rhabdomyoma.
11. Distinguish cardiac tumor from thrombus on CT and MRI.
12. Name the most common malignancies to metastasize to the heart; describe their appearance on chest radiography and CT.
13. Describe the anatomy of the coronary arteries, and identify the following on chest CT:
• Right coronary artery
• Left main coronary artery
• Left anterior descending coronary artery
• Left circumflex coronary artery
14. Identify on chest CT and describe the clinical significance of aberrant coronary artery origins.
15. Describe the clinical significance of coronary arterial calcification on a chest radiograph.
16. Recognize coronary arterial calcification on CT, and describe the current role of coronary artery calcium scoring with CT.
17. Identify the following postoperative findings on chest radiography and CT:
• Coronary artery stents
• Coronary artery grafts (normal and aneurysmal)
• Cardiac valve replacement
• Poststernotomy infection
18. Describe the difference between a left ventricular aneurysm and pseudoaneurysm, and distinguish between the two on chest CT.
19. Recognize pericardial calcification on a chest radiograph and CT, and name the most common causes.
20. Identify and describe two chest radiographic signs of a pericardial effusion.
21. Name five causes of a pericardial effusion.
22. Identify and describe the findings of each of the following on a chest radiograph and CT:
• Constrictive pericarditis
• Pericardial metastases
• Pneumopericardium
23. Describe the role of MRI in diagnosing constrictive pericarditis and differentiating constrictive pericarditis from restrictive cardiomyopathy.
Heart disease is the leading cause of morbidity and mortality in industrialized countries. Cardiac catheterization with cineangiography has long been used to assess the anatomy and function of the normal and diseased heart. Advanced technology has increased the options for noninvasive imaging, especially with CT, PET/CT, and MR. Echocardiography is often used to evaluate heart failure and valvular function. Exercise treadmill testing and nuclear stress testing are commonly used to evaluate patients with symptoms of possible cardiac origin. CT can be used to evaluate coronary artery calcification as a predictor of future risk of cardiovascular disease, coronary anatomy (particularly stenoses and anomalies of origin), and bypass grafts, and for pre-operative planning prior to ablation procedures. An added benefit of CT is the ability to globally assess the heart and lungs simultaneously. Cardiac MR is most commonly used to evaluate patients with symptoms of possible cardiac origin and causes of cardiomyopathy, and to evaluate cardiac anatomy after a heart attack or prior to ablation procedures. Assessment of cardiac morphology and function is usually performed with echocardiography or cardiac MR. CT is limited in individuals with fast or irregular heart rates or who cannot receive intravenous contrast. MR is limited in patients with certain implanted metallic objects (e.g., most pacemakers and all defibrillators) or who cannot receive gadolinium contrast.
Limiting content of this chapter to the essentials precludes a comprehensive discussion of coronary CT angiography, advanced morphology and functional evaluation of the heart, cardiac interventions, and recent innovations (e.g., myocardial perfusion imaging and targeted contrast agents). Cine imaging, which best illustrates aberrant cardiac function, is also not provided. This chapter will focus on the basic chest radiographic and CT findings of common congenital cardiac diseases seen in the adult, normal and abnormal cardiac anatomy, valvular disease, cardiomyopathies, cardiac tumors, complications of myocardial infarction and cardiac surgery, and pericardial disease.
CONGENITAL CARDIAC DISEASE
Whereas congenital abnormalities are the predominant form of heart disease in childhood, in adults, congenital disorders represent only 1% of recognized heart disease (1). In general, adults with newly discovered congenital heart disease are those whose anomalies produced no symptoms or mild symptoms in childhood, or those who were misdiagnosed in childhood only to become symptomatic in adult life. A discussion of all congenital heart defects that can be diagnosed in adults is beyond the scope of this chapter. What will be presented is a brief discussion of common left-to-right shunts that are diagnosed in adulthood and the developmental anomalies of the aorta that can be associated with congenital heart diseases.
Atrial Septal Defect
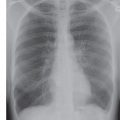
Atrial septal defect (ASD) accounts for 80% to 90% of congenital left-to-right shunts found in adults (2,3) and is three times more common in women than in men. Almost half of the patients with ASD in all age groups are asymptomatic, and in these cases the ASD is discovered incidentally on a routine chest radiograph. There are different types of ASD, with the ostium secundum type (consisting of an absence or deficiency of tissue in the region of the fossa ovalis) being the most common type seen in adult patients. In uncomplicated ASD, the chest radiograph shows enlargement of the right ventricle and all segments of the pulmonary arteries (“shunt vascularity”) (Fig. 18.1). The right atrium is also enlarged, but it is usually not distinguished from right ventricular enlargement on the chest radiograph. Because the radiograph does not accurately reflect the size of the right atrium and ventricle, the overall heart size may appear normal. On the lateral view, right ventricular enlargement results in a filling in of the retrosternal clear space and posterior displacement of the left ventricle toward the spine (4). There is no enlargement of the left heart in a simple ASD. The aorta appears small, relative to the pulmonary artery, and the superior vena cava appears small or “absent” because of rotation of the heart from right-sided cardiac enlargement (Fig. 18.2).
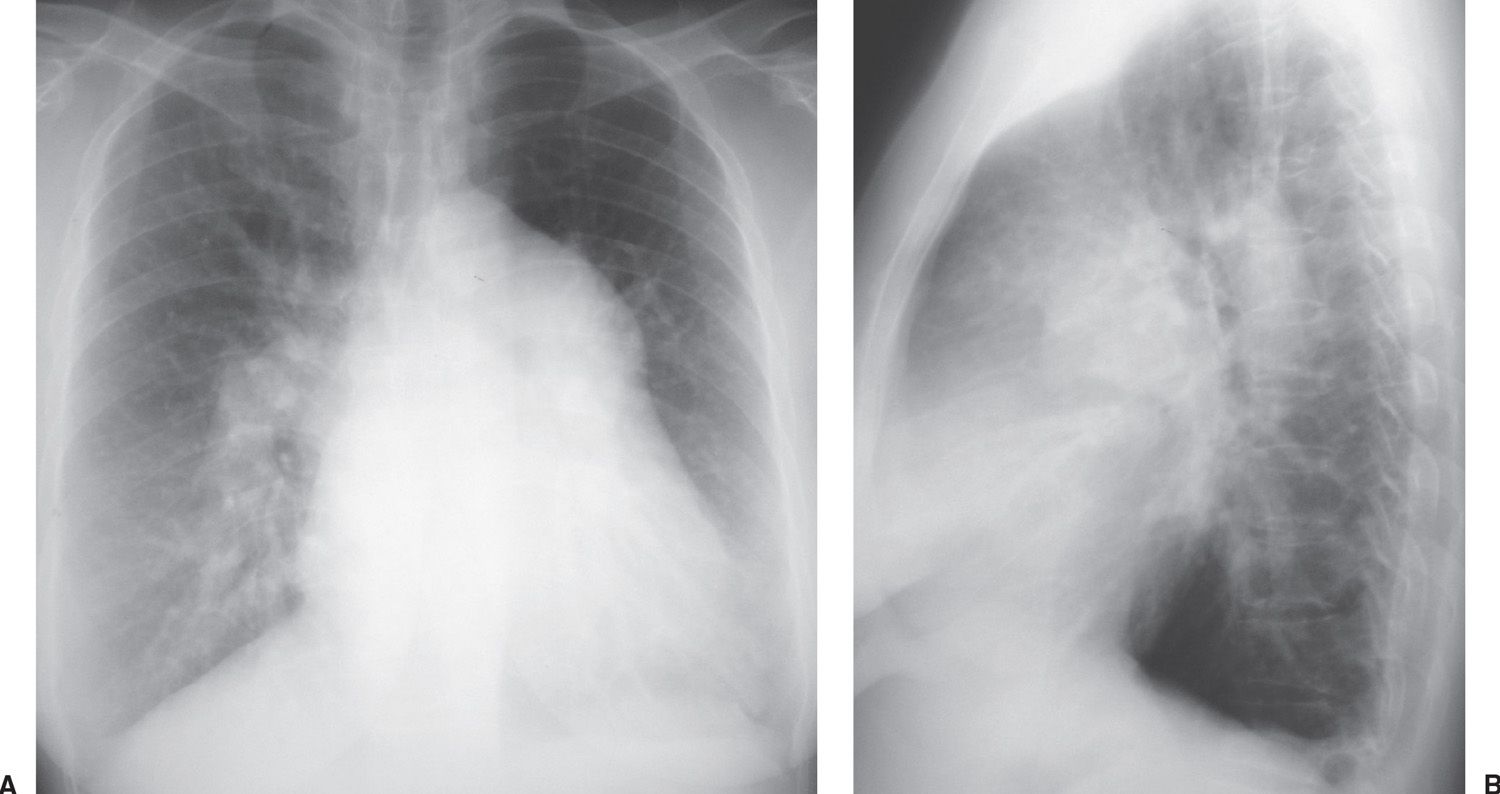
FIG. 18.1 • Atrial septal defect. A: Posteroanterior (PA) chest radiograph shows marked enlargement of the central and all segments of the pulmonary arteries. The cardiac silhouette is enlarged. B: Lateral view shows filling in of the retrosternal clear space, secondary to right ventricular enlargement, and pulmonary artery enlargement.
Long-standing large shunts lead to pulmonary arterial hypertension; when pulmonary arterial pressure exceeds systemic arterial pressure, a reversal of shunting of blood from left-to-right to right-to-left occurs (Eisenmenger physiology). In these cases, there is marked central pulmonary artery dilatation and narrowing of peripheral pulmonary artery branches (5). The central pulmonary arteries can become aneurysmal and, rarely, can be calcified (Fig. 18.3).
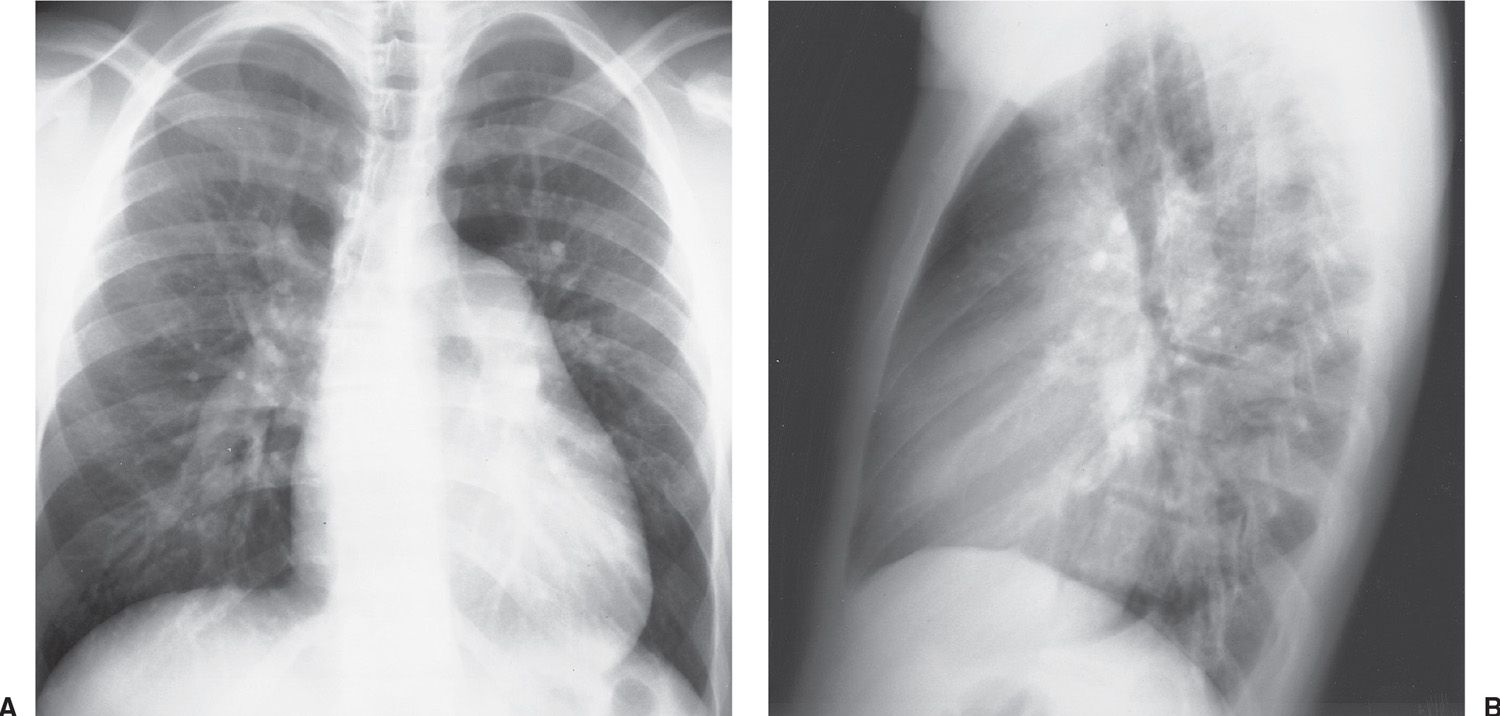
FIG. 18.2 • Atrial septal defect. A: PA chest radiograph of a 17-year-old boy with a heart murmur since birth shows enlargement of the cardiac silhouette, enlarged central and peripheral pulmonary arteries (“shunt vascularity”), a normal- to small-sized aorta, and “absent” superior vena cava shadow. B: Lateral view shows enlargement of the right ventricle, as evidenced by increased opacification posterior to the sternum.
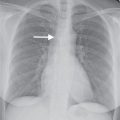
ASD closure can be accomplished with a variety of percutaneously placed devices. A catheter that is placed via the common femoral vein is fed through the cardiac defect into the left atrium using fluoroscopic and echocardiographic guidance. A commonly used device is the Amplatzer Septal Occluder (AGA Medical Corporation, Golden Valley, MN) (Fig. 18.4). This is a two-part closure device. One of two nitinol mesh disks is pushed out through the catheter into the left atrium; this is followed by release of the second disk into the right atrium to close the defect.
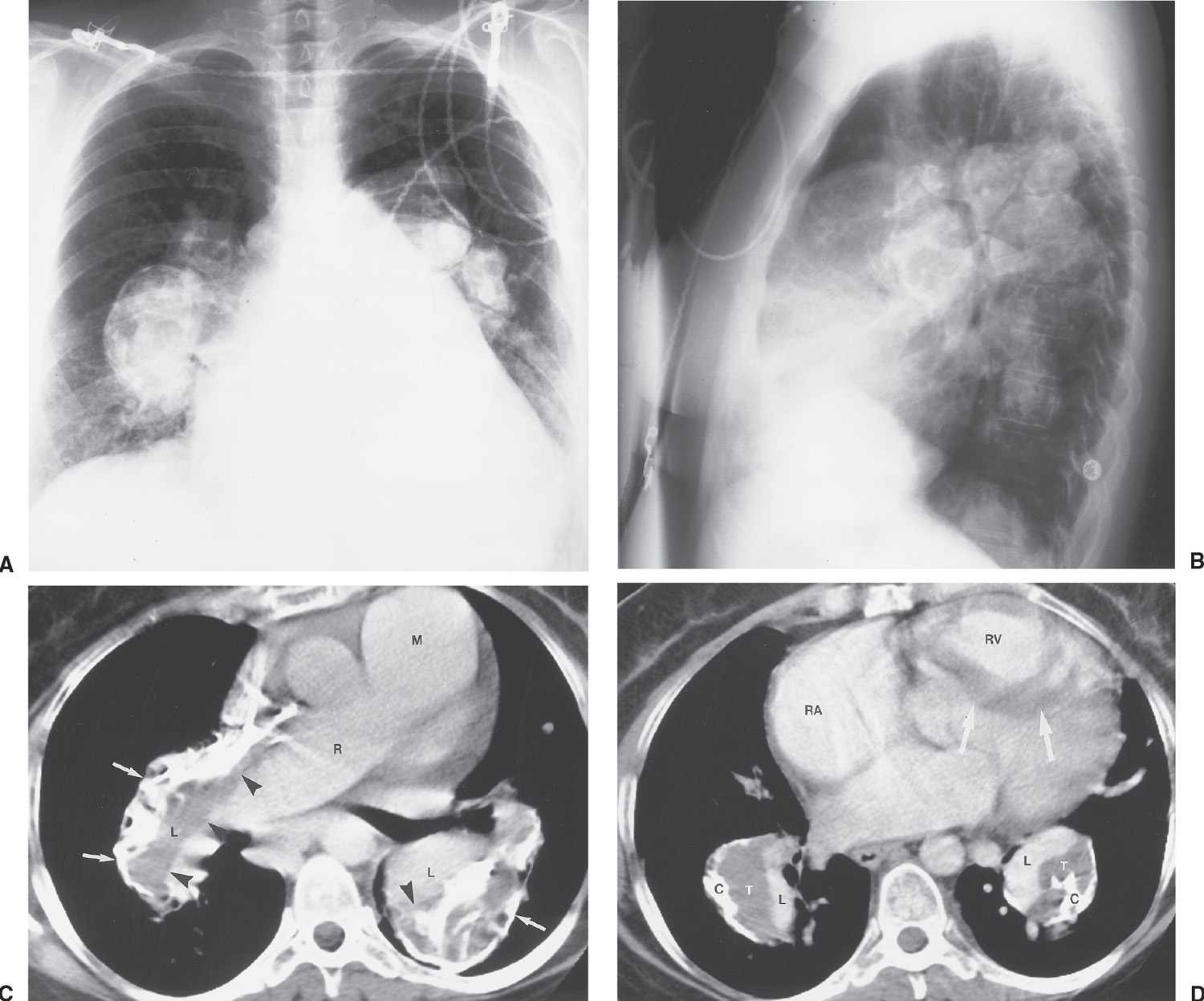
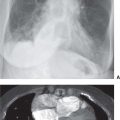
FIG. 18.3 • Atrial septal defect with eisenmenger physiology. PA (A) and lateral (B) chest radiographs of a 52-year-old woman with a large, long-standing ASD that has resulted in a reversal of shunting of blood and pulmonary arterial hypertension. There is aneurysmal enlargement and calcification of the central pulmonary arteries, enlargement of the right heart, and “absence” of the superior vena cava shadow. C: CT shows large main (M), right (R), and lower (L) lobe pulmonary arteries. Long-standing left-to-right shunting of blood has resulted in pulmonary artery aneurysms, which contain low-attenuation thrombus (arrowheads), and calcification (arrows). D: CT at a level inferior to (C) shows enlarged lower lobe pulmonary arteries containing thrombus (T) and calcification (C), enlarged right atrium (RA), and enlarged right ventricle (RV), causing leftward bowing and hypertrophy of the interventricular septum (arrows). The pulmonary artery lumina are outlined by high-attenuation contrast material (L).
Ventricular Septal Defect
Ventricular septal defect (VSD) is the most common congenital heart disease in childhood, but it represents only 10% of congenital cardiac lesions in the adult patient. Surgical correction and spontaneous closure of the defect account for the decreased incidence in the adult patient (6). Most patients with VSD who survive into adulthood without intervention have small and physiologically inconsequential defects. Patients who reach adult life with large VSDs have pulmonary arterial hypertension, progressive right-to-left shunting (Eisenmenger physiology), and cyanosis. Small shunts cannot be identified on chest radiography. With large shunts (VSDs with a shunt ratio greater than 2:1) and normal pulmonary vascular resistance, the chest radiograph shows “shunt vascularity,” enlargement of the left atrium and both ventricles, a normal or small aorta, and a normal right atrium. The left ventricular apex projects to the left, inferiorly and posteriorly. On frontal chest radiographs, enlargement of the left atrium is seen as a “double density” behind the right atrium, and straightening or focal convexity of the left mediastinal border is seen below the pulmonary artery shadow.
Patent Ductus Arteriosus
The ductus arteriosus is a portion of the sixth aortic arch in the fetus that connects the left pulmonary artery to the descending thoracic aorta. Twelve to 24 hours after birth, the ductus is functionally closed, and anatomic closure occurs 1 to 2 weeks later. However, for unknown reasons, the ductus arteriosus may remain patent, creating a left-to-right shunt and varying degrees of overcirculation to the lungs, left atrium, left ventricle, and the ascending and arch portions of the aorta. If the ductus does not close, Eisenmenger physiology develops when pulmonary vascular resistance exceeds systemic resistance and causes a right-to-left shunt across the ductus arteriosus. The radiographic features of an uncomplicated patent ductus arteriosus (PDA) are similar to those of a VSD, except for the size of the aorta. In PDA, the ascending and arch portions of the aorta can enlarge (with the degree of enlargement dependent on the size of the shunt), indicating that the shunt is extracardiac, as opposed to an intracardiac shunt, such as a VSD, with which the size of the aorta is normal or small. However, aortic size is not always a reliable criterion, and in practice it can be difficult to distinguish VSD from PDA on chest radiography. Another finding of PDA in an adult is calcification of the ductus.
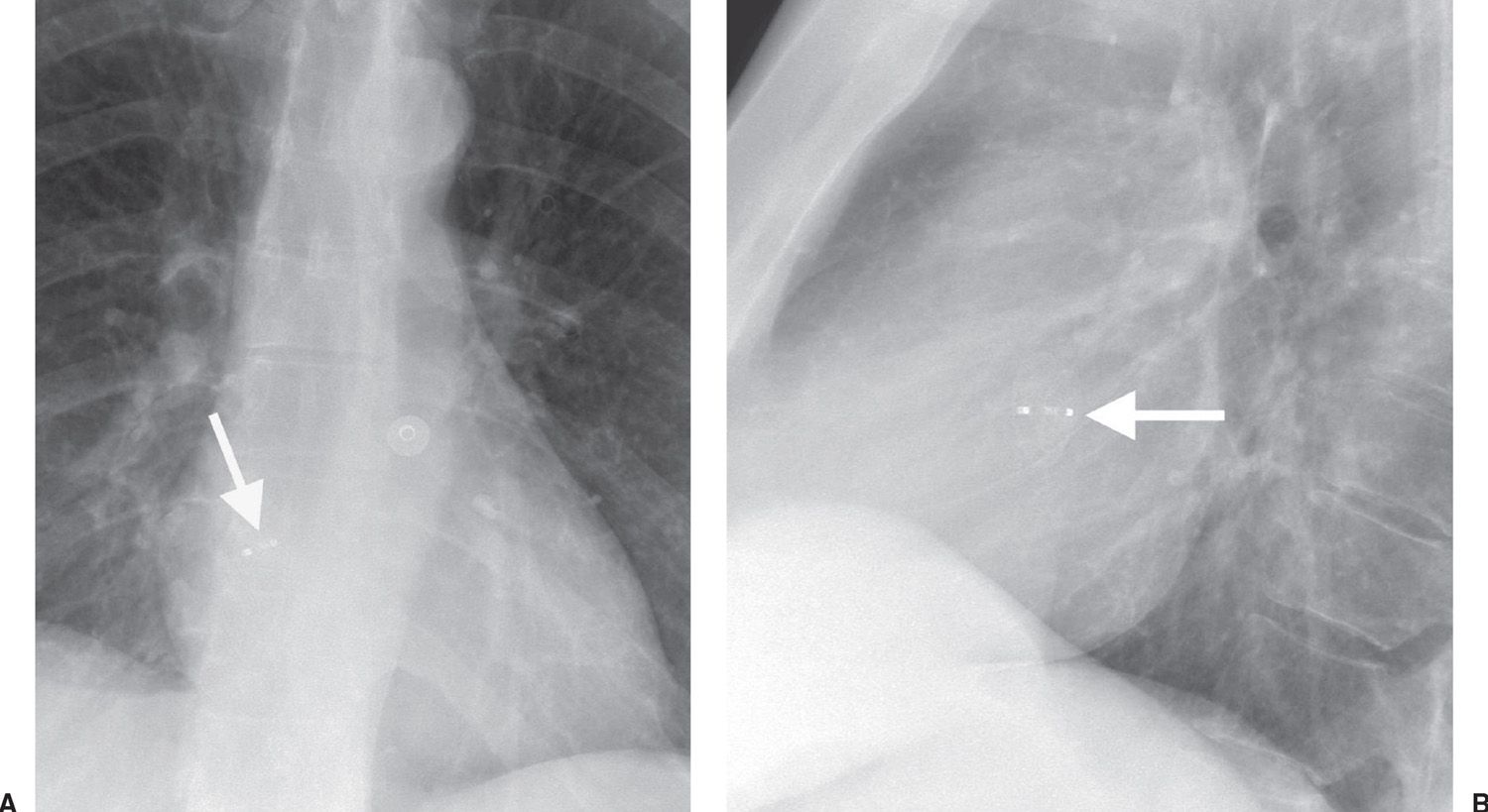
FIG. 18.4 • Atrial septal defect occluder. A: PA chest radiograph of a 50-year-old woman with a history of transient ischemic attacks shows an Amplatzer Septal Occluder device (AGA Medical Corporation, Golden Valley, MN) (arrow) in the location of the foramen of ovale. B: Lateral view confirms appropriate placement of the device (arrow).
Anomalies of the Thoracic Aorta
Congenital bicuspid aortic valve is a relatively common malformation. As the valve thickens and becomes fibrotic, it becomes stenotic. When this occurs, the valve becomes calcified. A densely calcified aortic valve, as seen on chest radiography or CT in a patient under the age of 55, should prompt consideration of this anomaly.
In left aortic arch with aberrant right subclavian artery, the right subclavian artery, instead of the first branch, takes off as the final branch of the aorta. Patients with this common anomaly are usually asymptomatic, but may present with dysphagia as a result of the retroesophageal course of the aberrant artery. The frontal chest radiograph often shows an abnormal mediastinal contour at the level of the aortic arch, where the proximal portion of the aberrant artery is dilated—the so-called diverticulum of Kommerell. The lateral chest radiograph may show anterior displacement of the trachea.
A right aortic arch occurs when there is interruption of the embryonic left aortic component of the hypothetical double aortic arch. Two types are described: Type 1 is a mirror image of left aortic arch and is associated with cyanotic congenital heart disease in more than 95% of patients, most of whom have tetralogy of Fallot (7). Type 2 right aortic arch—a right aortic arch with an aberrant left retroesophageal subclavian artery—is common, occurring in 1 in 2,500 persons, and is usually found incidentally (1). Additional cardiac anomalies occur in only 5% to 15% of patients with type 2. Chest radiographs of a right aortic arch show that the trachea is bowed to the left at the level of the right aortic arch, producing a convex bulge just above the azygos vein (Figs. 18.5 and 18.6). It is typical for a right-sided arch to be high riding and present as a “mass” in the right paratracheal area. An aortic diverticulum, or proximal arterial dilation, is seen to the left and slightly below the usual site of a left-sided aortic arch. The diverticulum may be of sufficient size to produce a large bulge immediately above the left main pulmonary artery and simulate a left aortic arch or a nonvascular mediastinal mass. Most often, the left mediastinal bulge is a result of the aortic diverticulum rather than the aberrant left subclavian artery itself. As the left subclavian artery crosses from right to left, it will cause a posterior impression on the air-filled esophagus and the trachea, which is seen best on the lateral chest radiograph. The right aortic arch usually crosses to the left side posteriorly, in the middle of the thorax behind the right pulmonary artery, to descend into the abdomen on the left side. A double aortic arch forms a complete vascular ring and on occasion can first present in adulthood.
Pseudocoarctation is a term used to denote a focal narrowing of the aortic arch that has the same morphology as classic coarctation but does not produce obstruction. This anomaly is a buckling of the aorta at the isthmus with little or no pressure gradient across the buckled portion (less than 30 mm Hg) (8). Because there is no obstruction, there is no collateral flow, and there is no rib notching, as is seen with classic coarctation. The chest radiograph shows a left “mediastinal mass” that represents an elongated, redundant, and high aortic arch. Sagittal CT reconstructions of the chest can show the high arch with a kink at the isthmus, resembling the numeral 3, in which the midportion of the 3 corresponds to the attachment of the ligamentum arteriosum (Fig. 18.7). A “cervical aortic arch” can resemble pseudocoarctation of the aorta on chest radiographs, but with a cervical arch, the aortic arch lies in the neck, above the clavicles, usually on the right side.
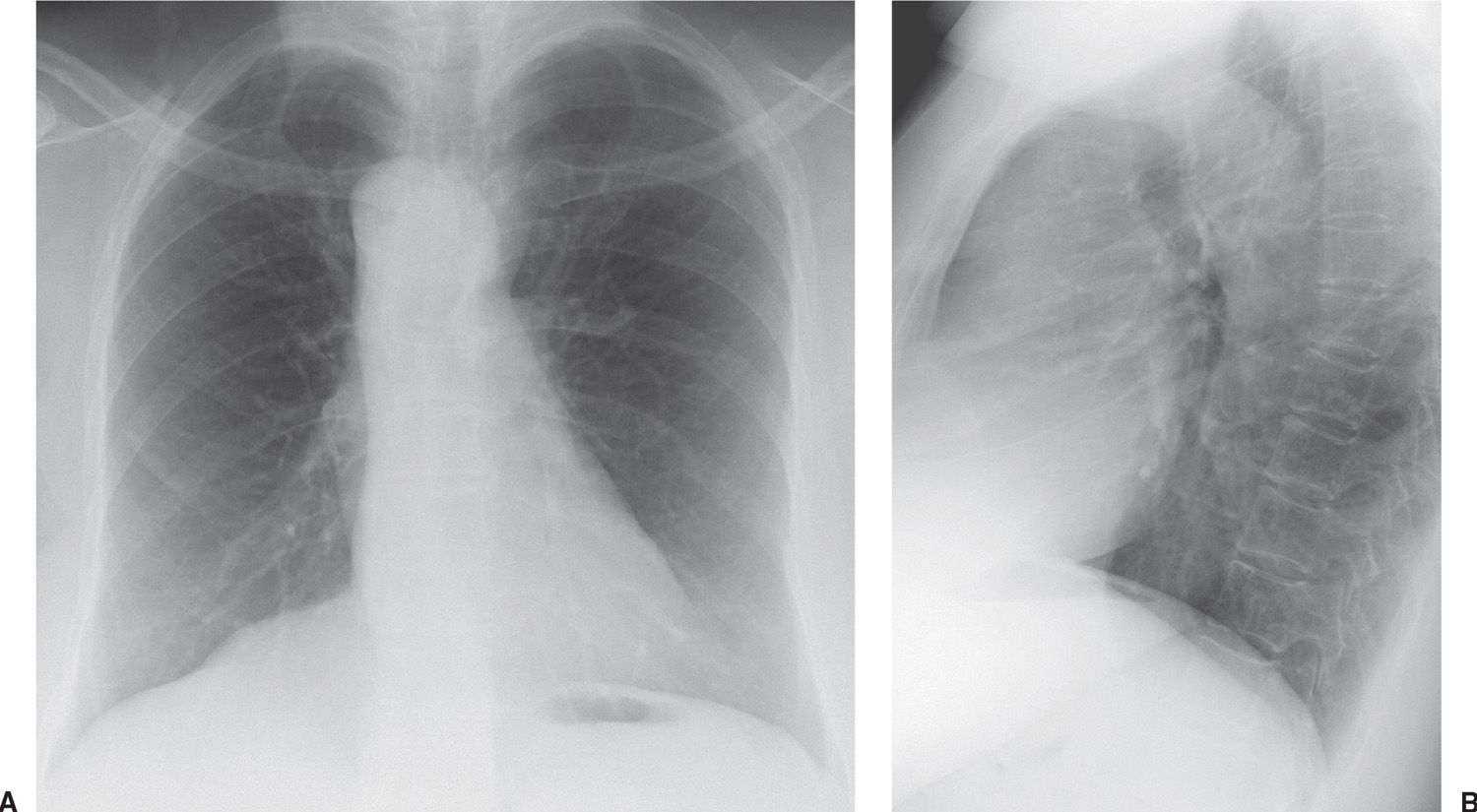
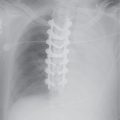
FIG. 18.5 • Right aortic arch. A: PA chest radiograph shows a right-sided aortic arch and descending aorta. The trachea is not deviated to the right, as is usually seen with a left aortic arch. B: Lateral view shows a posterior impression on the tracheal air column, secondary to compression from the aberrant left subclavian artery crossing from right to left.
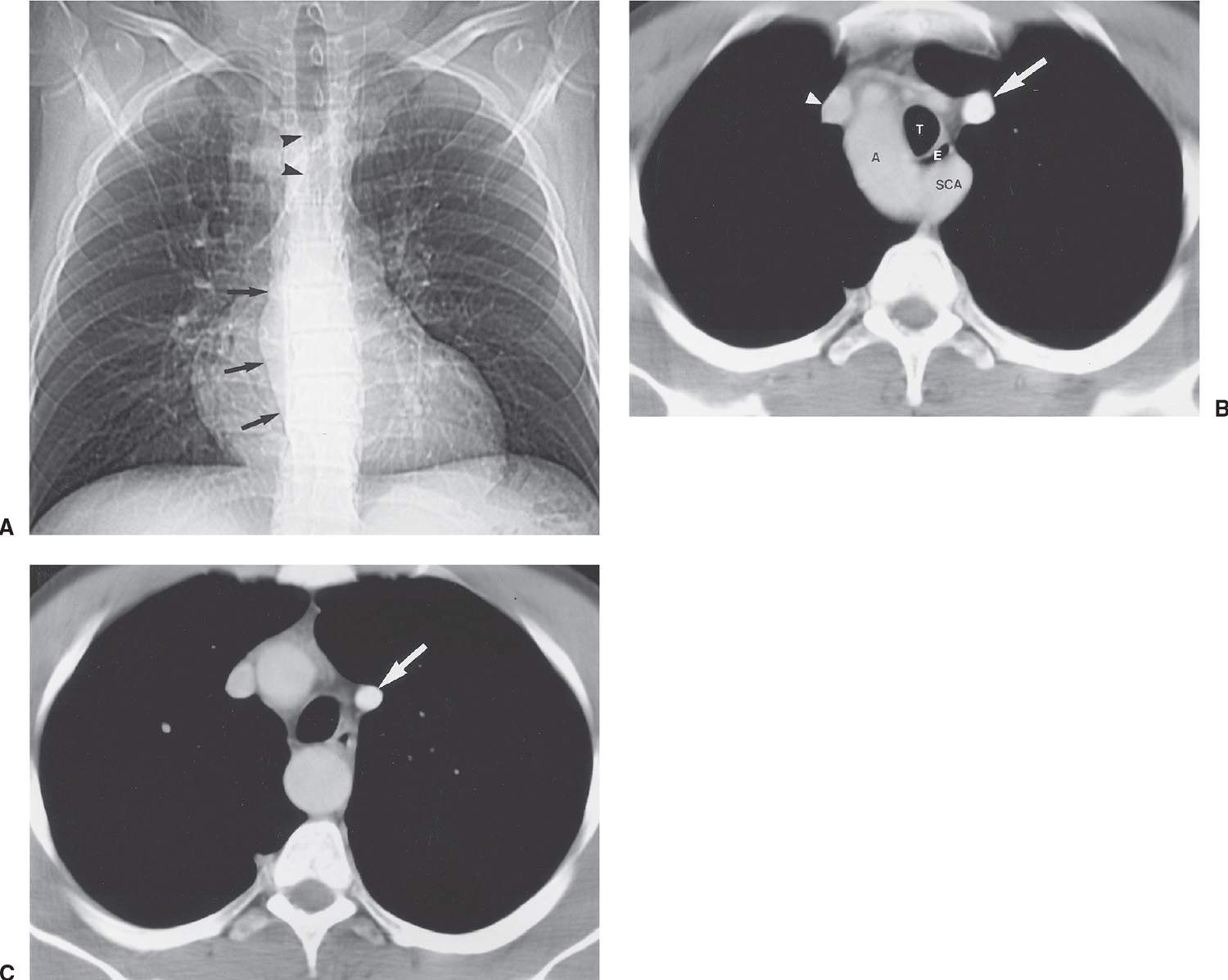
FIG. 18.6 • Right aortic arch with aberrant left subclavian artery. A: Scout view from a CT of a 29-year-old man with dysphagia shows deviation of the trachea to the left (arrowheads) and a right-sided descending thoracic aorta (arrows). B: CT shows the right-sided aortic arch (A) and an aberrant left subclavian artery (SCA) arising from the posterior arch and coursing posterior to the trachea (T) and esophagus (E). Note the compression of the esophagus from the aberrant vessel, causing the patient’s dysphagia. There is also a persistent left superior vena cava (arrow), in addition to a right superior vena cava (arrowhead). C: CT at a level inferior to (B) shows the persistent left superior vena cava (arrow) coursing in a left paramediastinal location before draining into the coronary sinus more inferiorly. The descending aorta is midline at this level.
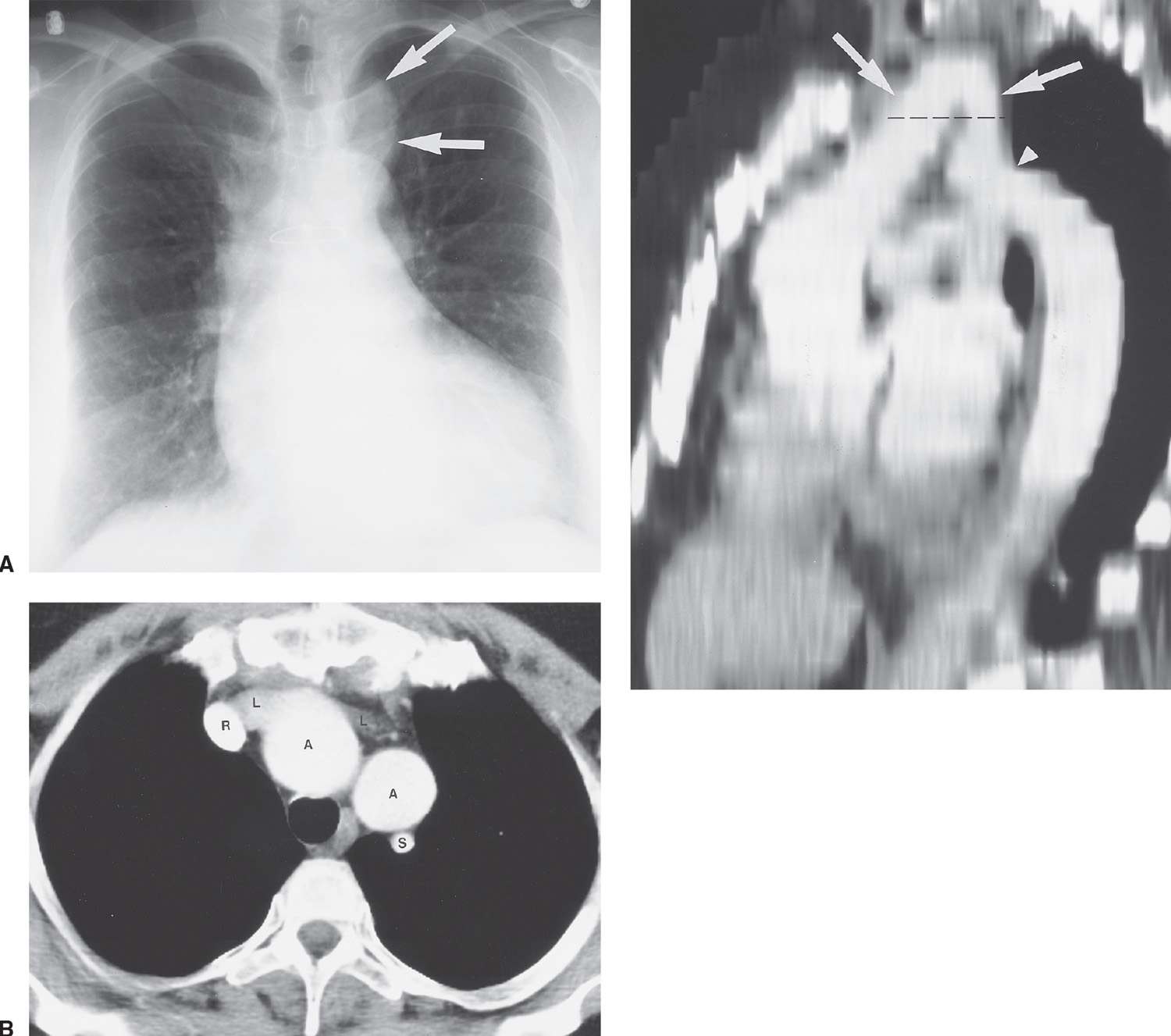
FIG. 18.7 • Pseudocoarctation of the aorta. A: PA chest radiograph of a 50-year-old woman shows a left paratracheal “mass” (arrows). Sternotomy wires are present from previous coronary artery bypass graft surgery. B: CT shows the left subclavian artery (S), right (R) and left (L) brachiocephalic veins, and the high-riding, “buckled” aortic arch (A). C: Sagittal reconstruction shows the buckled aortic arch (arrows) and focal kinking of the aorta at the isthmus (arrowhead). This reconstruction shows how an axial view at the level of the aortic arch (dashed line) will show “two” rounded, contrast-enhanced structures adjacent to each other.
ACQUIRED HEART DISEASE
Valvular Disease
The radiologic signs of uncomplicated valvular lesions are relatively straightforward (Table 18.1). Valve stenosis produces pressure overload and myocardial hypertrophy without dilatation. Dilatation indicates that heart failure has developed. Valve insufficiency produces volume overload and a combination of dilatation and hypertrophy of the involved cardiac chambers. With insufficiency, a dilated heart does not indicate cardiac decompensation.
Aortic Stenosis
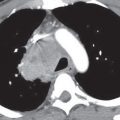
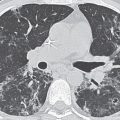
Aortic stenosis (AS) can exist at the valvular, subvalvular, or supravalvular level. The chest radiographic abnormalities depend on the age of the patient as well as on the severity of the stenosis. The adult heart is of normal size, and the lungs are normal, because left ventricular failure and dilatation occur only in terminally ill patients. Radiographically detectable calcification in the aortic valve occurs in all types of AS and marks the stenosis as clinically severe (Figs. 18.8 and 18.9). Dilatation of the ascending aorta is frequent in AS but correlates poorly with severity or with the site of the stenosis. Poststenotic dilatation of the ascending aorta is caused by the jet of blood through the stenotic valve striking the lateral aortic wall. The lateral wall of the aorta becomes both dilated and elongated, accentuating the rightward displacement of the aorta. In most children and adults with pure, severe AS, the left ventricle is a small cavity that is hypercontractile and has the usual signs of hypertrophy (Fig. 18.10). In the absence of other anomalies, left ventricular dilatation in pure AS is direct evidence of heart failure.
Calcific AS in the adult can be caused by rheumatic heart disease, a congenital bicuspid aortic valve, or advanced age and degeneration of the valve (8). The average age at which aortic valve calcification is first detected is 25 years for congenital AS, 47 years for rheumatic AS, and 54 years for degenerative AS (9). Valve calcification is best seen on the lateral chest radiograph, because the valve usually projects over the spine in the frontal projection. An important clue to the diagnosis of rheumatic disease in the aortic valve is the presence of mitral stenosis or regurgitation.
Table 18.1 RADIOGRAPHIC APPEARANCES OF CARDIAC VALVULAR DISEASE
Valvular Disease | Radiographic Findings |
Aortic stenosis | • Calcification of aortic valve • Dilatation of ascending aorta • Dilated left ventricle and pulmonary edema only with left ventricular failure |
Aortic insufficiency | • Calcification of aortic valve • Dilatation of ascending aorta • Dilated left ventricle • Pulmonary edema with left ventricular failure |
Mitral stenosis | • Enlargement of left atrium, right ventricle, and pulmonary trunk • Cephalization of pulmonary vasculature • Septal (Kerley B) lines • Calcification of mitral valve |
Mitral insufficiency | • Markedly dilated left atrium • Mildly dilated left ventricle • Moderate enlargement of pulmonary trunk and right ventricle |
Tricuspid stenosis | • Systemic venous dilatation • Pulmonary oligemia |
Tricuspid insufficiency | • Dilatation of right ventricle and right atrium • Dilatation of venae cavae • Pulmonary oligemia |
Pulmonic stenosis | • Right ventricular enlargement • Poststenotic dilatation of the pulmonary trunk and left pulmonary artery • Increased pulmonary blood flow to the left lung and decreased pulmonary blood flow to the right lung |
Pulmonic insufficiency | • Dilatation and hypertrophy of the right ventricle • Systolic enlargement of the pulmonary trunk and central pulmonary arteries |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree