Daniel C. Brown • Constantino S. Peña • James F. Benenati
BACKGROUND
Visceral abdominal aneurysms (VAAs) are rare, accounting for less than 1% of all arterial aneurysms, but can be life threatening, with reports of morbidity and mortality ranging up to 100% in the setting of aneurysm rupture.1,2 The treatment options vary depending on the vessels involved, aneurysm type (true aneurysm or pseudoaneurysm), etiology, and size.
The incidence of VAAs has been reported to be between 0.1% and 2% of the population, although autopsy studies have demonstrated much higher rates, up to 10%.3–5 An increased incidence in visceral aneurysms in recent years likely corresponds to the detection of asymptomatic aneurysms seen on the simultaneously increased number of cross-sectional imaging (computed tomography [CT] and magnetic resonance imaging [MRI]) examinations obtained over this time. Additionally, the higher prevalence of interventional procedures in the treatment of various abdominal pathologies has led to a concomitant increase in the incidence of iatrogenic pseudoaneurysms.1,3,4
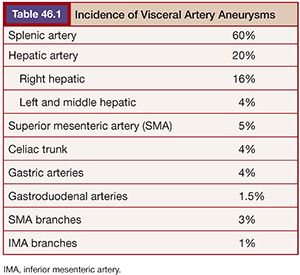
True aneurysms are defined as a vessel diameter expansion of at least 50% that involves all three vessel wall layers: the intima, media, and adventitia.3 A pseudoaneurysm (or false aneurysm) fails to involve all three vessel layers but instead represents a form of vessel wall disruption allowing a plane of blood flow that is usually only contained by the adventitia3 or surrounding tissues, explaining its higher mortality compared to true aneurysms. Pseudoaneurysms (PAs) are usually iatrogenic or related to trauma and inflammatory conditions such as pancreatitis.6 Unfortunately, determining the type of aneurysm is not always possible from imaging characteristics alone, requiring a complete evaluation of the patient’s history, physical, and other vascular anatomy. The splenic (60%) and hepatic (20%) arteries account for the largest distribution of visceral aneurysms followed by the superior mesenteric (5%) and celiac (4%) arteries3,7 (Table 46.1). Interestingly, more than one visceral aneurysm is found in greater than a third of patients.
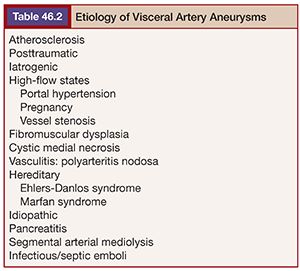
The possible etiologies of visceral aneurysms can be extensive (Table 46.2). However, attempting to determine their specific cause may be helpful in establishing the risk of rupture and developing successful treatment options. Degenerative and atherosclerotic aneurysms are commonly seen in the splenic, celiac, and superior mesenteric arteries. The presence of vessel wall calcifications usually suggests a stable, chronic process, whereas eccentric aneurysms without thrombus or calcification may be more concerning for rupture. In the setting of inflammatory or infectious processes, it may be best to ameliorate the process with systemic therapies before proceeding with nonemergent therapy.
As with other peripheral aneurysms, the strict use of a size criteria to guide the decision to treat is controversial and likely too simplistic. There are several factors that should determine the need for treatment. Several nonspecific guidelines have reported the use of a 2-cm threshold for the treatment of large vessel, asymptomatic visceral aneurysms such as those associated with the hepatic and splenic arteries.8,9 However, the decision to treat and how to do so should likely include many factors, including the patient’s symptoms, aneurysm location and type, etiology, presence of calcification or thrombus, and particular visceral vessel hemodynamics. The potential treatment options will be based on these factors, and the possible complications from those various treatment options must also be considered. Generally speaking, PAs and symptomatic aneurysms should be treated regardless of their size. In contrast, over the last decade, most interventionalists have chosen to follow stable true visceral aneurysms that are less than 2.5 cm in diameter and lack concerning features, such as interval growth, which is considered a reason for treatment. It must be stated that the data on when to treat VAA is based on a limited experience primarily derived from single center and retrospective evaluations of ruptured aneurysms over the last three decades.
Treatment Options
Given the high morbidity and mortality observed with visceral aneurysm rupture, treatment to prevent rupture is paramount. Traditionally, surgical management was the primary treatment option, whereas endovascular options were initially only considered when patients were deemed too high risk for surgery. However, endovascular treatment offers a multitude of therapeutic options, with high technical success rates and minimal morbidity and mortality.10–12 As a result, endovascular treatment is now the first option, with surgery typically reserved for instances of acute large vessel ruptures, rebleeding, or growth after intervention and aneurysms that are not amenable to primary or repeat endovascular therapy.
VAA treatment should begin with a careful assessment of the patient’s history and physical examination to assess the etiology and chronicity of the aneurysm. The evaluation of cross-sectional imaging is also crucial for planning a successful treatment strategy. Computed tomography angiography (CTA) and magnetic resonance angiography (MRA) evaluation allows for the assessment of an aneurysm neck and the number and size of afferent and efferent vessels. The amount and extent of at-risk end organ tissue should also be evaluated. The presence of other visceral vessel stenosis and collateral pathways should be considered in deciding possible embolization techniques, locations, and agents. Additionally, thought and attention should be made to the endovascular access and treatment vessels to help determine potential treatment options, including puncture site location (femoral, upper extremity, direct puncture), sheath, catheter and microcatheter size, landing zone, coil or plug size, and delivery. Along with standard axial images, we also reformat CTA into thick (12 × 2 mm) maximum intensity projections (MIPs) in coronal and sagittal planes or used real time three-dimensional volume–rendered reconstructions to aid us in making these decisions before undertaking treatment.
Devices and Techniques
At an elementary level, VAA treatment involves prevention of subsequent aneurysm rupture by stopping blood flow to the aneurysmal or weakened portion of the artery. Treatment techniques attempt to prevent or minimize downstream or end organ effects by preserving collaterals or some form of end organ perfusion.1 The use of embolization coils to block flow has been the primary treatment tool. Recent advances include retrievable and gel expanding coils that achieve great success in obstructing inflow and outflow vessels or in the filling of an aneurysm sac with coils. Additionally, metallic expandable plugs have been used to similarly block efferent and afferent vessels.13 The use of liquid embolics (N-butyl cyanoacrylate [NBCA (glue)] and ethylene vinyl alcohol [Onyx; Covidien, Irvine, California]) to fill and mold to the size of the aneurysm sac has also been a useful technique in excluding flow to the aneurysm sac. However, although effective, these liquid embolics require delivery in an extremely controlled fashion.14 In VAA, the collateral pathways can be a double-edged sword that may protect end organ flow but may also revascularize the aneurysm by providing collateral or backflow into the aneurysm from other vessels that were not initially evident. For this reason, careful evaluation of VAA hemodynamics must be performed. Additionally, these pathways may limit the use of particle embolics as the potential for end organ damage may be great.
The development of covered stents has allowed for the possibility of preventing vessel rupture by strengthening or reinforcing the weakened vessel segment but also allowing continued end organ flow. These devices allow for the traditional blood flow patterns to continue within the visceral vessels. Unfortunately, the size of the covered stent delivery systems relative to the treatment vessel size as well as vessel tortuosity have limited their application to proximal splenic and hepatic arteries. Additionally, the vessel diameter proximal and distal to the aneurysm must be relatively equivalent.7 Stent-assisted coiling can also be performed to treat VAA. This is a technique that uses a bare metal stent by placing it across an aneurysm neck where it serves as a support or scaffold through which a catheter is then used to deploy coils into the aneurysm. The bare stent preserves distal flow while the coils are positioned through and around the stent into the aneurysm sac.
SPLENIC ARTERY ANEURYSMS
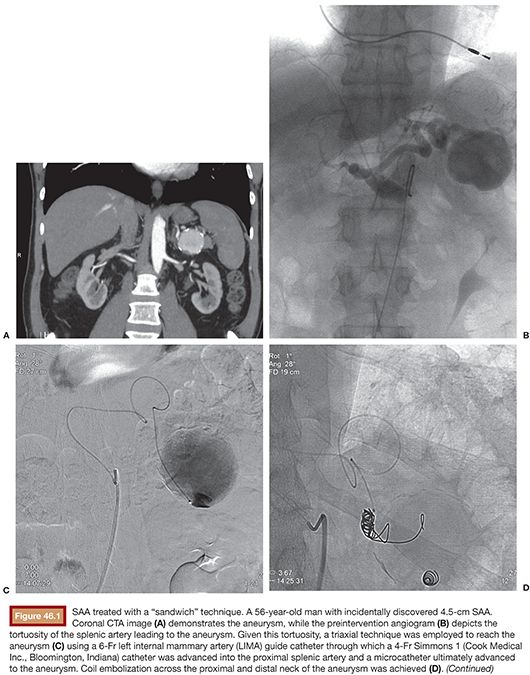
Splenic artery aneurysms (SAAs) are the most common true visceral artery aneurysm. They are most often saccular and located in the mid to distal splenic artery. The etiology of true SAA is varied including atherosclerosis, high-flow states such as pregnancy and portal hypertension, and liver transplantation.4,15,16 Traditionally, the splenic artery represents the single inflow and the single outflow vessel. This allows for coil embolization of the outflow segment first, followed by the inflow segment, a technique known as the sandwich technique, the isolation technique, or the coil-trapping technique. This may or may not also involve coil packing of the aneurysm sac itself (Fig. 46.1). This technique can also be performed with vascular plugs. The potential for collateral flow back to the SAA should be excluded by occluding both the inflow and outflow vessels adjacent to the aneurysm.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree