Krishna Kandarpa
Surgically unresectable metastases confined to the liver are the life-limiting component of disease for many patients with melanoma, whether ocular or cutaneous in origin. Hepatic metastases are present in 40% to 50% of patients at initial diagnosis with ocular melanoma, and there is subsequent liver involvement in up to 95% of individuals who develop ocular melanoma metastases.1 Ocular melanoma, although rare, is the most common primary ocular malignancy in adults. The mean age-adjusted incidence of ocular melanoma in the United States is approximately 4.3 new cases per million population per year.2 However, liver involvement occurs in only about 15% to 20% of patients with metastatic cutaneous melanoma.3,4 Median survival for patients with hepatic melanoma metastasis is less than 6 months. Most therapies, whether systemic or regional, have low response rates and limited efficacy.5–8
New systemic therapies have recently been approved for cutaneous melanoma (i.e., ipilimumab and vemurafenib). However, ipilimumab is only effective in 25% of cutaneous melanoma patients and it often has a delayed time to response of 3 to 6 months, which is too long to benefit many patients.9 Vemurafenib is an option for only the 50% of patients with cutaneous melanoma who have BRAF mutations, but almost all of these patients relapse within 6 to 8 months. Because BRAF mutations are not observed in patients with ocular melanoma, vemurafenib is not an option for them, and immunologic therapies, such as ipilimumab, have not shown benefit to date.
Surgical resection of melanoma liver metastases is limited by their numbers and location and is the only potentially curative option, albeit in fewer than 10% of patients.10 As there is currently no treatment that can alter the course of the disease, there remains a critical unmet need for these patients.
RATIONALE FOR PERCUTANEOUS HEPATIC PERFUSION
Intense nonsurgical regional treatment of the liver aims to provide maximal efficacy while limiting systemic exposure to toxic drug effects. Focal cryotherapy, radiofrequency heating, or ethanol injection are approaches to ablating tumors while minimizing injury to surrounding normal liver. Lesion visibility, numbers, size, and the proximity to vital structures limit the use of these treatments.11 They are a suboptimal option if occult micrometastases are present.
The liver possesses unique anatomic characteristics that allow it to be completely isolated from the rest of the systemic circulation, facilitating high-dose infusion of chemotherapy to the liver via the hepatic artery. Unlike local ablation or embolization that only treat selected visible tumors, hepatic perfusion treats the entire liver, including occult micrometastases, potentially limiting recurrence and improving efficacy while minimizing systemic drug exposure and toxicity. An open-abdominal surgical approach known as isolated hepatic perfusion (IHP) isolates the liver’s vasculature and creates a closed circuit within which a high dose of melphalan is circulated though the liver parenchyma. The small volume of blood confined within the circuit never mixes with the systemic circulation and is discarded after the procedure. IHP has been reported to control disease and extend survival for patients with unresectable hepatic metastases.12–16 IHP has had limited adoption because of its technical complexity, invasiveness, high morbidity, and the development of intra-abdominal adhesions that limit it to a one-time-only treatment.
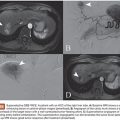
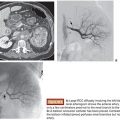
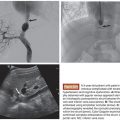
In part to overcome the limitations of IHP, percutaneous hepatic perfusion (PHP) was developed as a minimally invasive alternative. It is an investigational drug (melphalan hydrochloride)/device combination product in the United States (not approved by the U.S. Food and Drug Administration [FDA]), but the device component has European Union CE mark approval to be used in combination with independently procured melphalan. The device consists of several sterile, single-use components, including catheters and an extracorporeal circuit with hemofiltration cartridges (Delcath Systems, Inc., New York, New York). A high dose of melphalan is delivered to the liver via a hepatic artery catheter, and the hepatic venous return is captured and filtered extracorporeally to minimize systemic exposure before it is returned to the systemic circulation (Fig. 42.1). This is achieved by creating an extracorporeal venovenous bypass circuit following adequate anticoagulation with heparin. A double-balloon catheter is placed across the retrohepatic inferior vena cava to isolate the hepatic venous blood. The procedure takes approximately 3 hours (in-room) and is performed under general anesthesia and uses standard interventional radiology techniques.
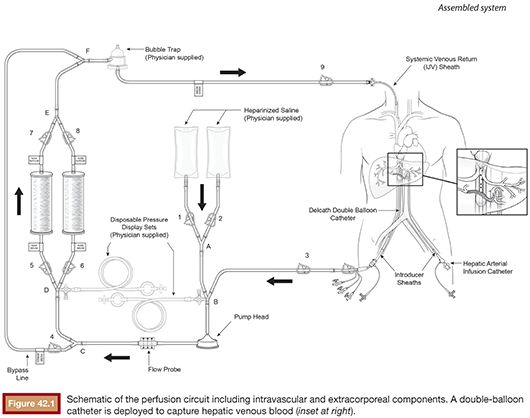
ROLE OF THE INTERVENTIONAL RADIOLOGIST
The decision to treat with PHP is generally made during interdisciplinary rounds that include oncologists, surgeons, interventional radiologists, and possibly other specialists. Once the decision is made to treat with PHP, the interventional radiologist becomes the designated procedural leader, but the oncologist remains the primary managing physician. The interventional radiologist manages the medical imaging and performs the necessary catheter placements and arterial embolization to redirect the vasculature as needed.16,17
Embolization is preferably completed during an earlier session at least a week ahead of the planned PHP procedure. Although embolization may be performed during the procedure, it is generally not advised as the intraprocedural use of large quantities of heparin may preclude adequate occlusion of the acutely embolized branches. This practice also allows the arterial access site to heal, if one does not use a closure device after embolization, and it reduces the duration of general anesthesia and the PHP procedure.
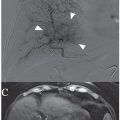
In addition to the other imaging required, the interventional radiologist must perform complete visceral angiography (celiac and superior mesenteric arteries with visualization of the portal vein to assess flow and patency). All gastrointestinal (GI) branches arising from the hepatic arteries are assessed for potential misperfusion, whether directly by antegrade flow or by retrograde reflux into branches proximal to the site of infusion. In an analysis of patients who randomized primarily to PHP in the phase 3 trial discussed in the following text, Stedman et al.18 reported 91 embolization procedures in 42 patients. These included 64 gastroduodenal, 12 right gastric, and 5 left gastric branches. An additional 10 embolizations included phrenic, supraduodenal, gastrohepatic, aberrant left gastric arising from hepatic, and other less common branches. Patients who crossed over from the control to the treatment arm (n = 28) had a similar pattern of embolization. Most embolizations were performed before the first PHP cycle, with far fewer performed before cycles 2 to 4 and none during cycle 5 or 6 (up to 6 cycles of PHP were allowed for qualifying patients). This practice of hepatic vascular “redistribution” is familiar to interventional radiologists, but unlike some other liver-directed therapies, lung shunting studies have not been needed.
On the day of PHP, the interventional radiologist places the femoral arterial, femoral venous, and internal jugular venous sheaths and catheters and actively directs the team during the procedure, working with the anesthesiologist and perfusionist (B. McCormick et al., unpublished data, 2014) to safely establish the extracorporeal (EC) circuit, to perform hepatic arterial infusion, and to terminate the EC circuit once the procedure is completed. The actual percutaneous hepatic perfusion procedure takes 1 hour: during the first half hour, melphalan is infused into the hepatic artery with simultaneous filtration, and during the second half hour, infusion is stopped, but an additional 30 minutes of washout filtration is continued. When the extracorporeal blood flow is initiated, profound hypotension can occur, and the anesthesiologist should manage the depth of the BP drops through the use of prophylactic corticosteroid and vasopressors as well as additional intraprocedural vasopressors as needed. The use of vasopressors may be accompanied by peripheral vasospasm. Thus, during the procedure, the interventional radiologist must check frequently for hepatic arterial vasospasm and relieve it using intra-arterial (IA) nitroglycerin. Stedman et al.18 reported a median IA dose of 230 µg (range 50 to 1,200 µg). Once the procedure is completed and EC circuit terminated, the anesthesiologist reverses the anticoagulation and provides the needed postprocedural support for the patient (C.M. Chen et al., unpublished data, 2014). The interventional radiologist removes the catheters and sheaths and works with the anesthesiologist and intensive care unit (ICU) physicians until the patient has recovered from procedure-related side effects overnight. The patient is moved to the general wards the next day at which time the oncologist takes over the subsequent management of the patient. Seamless communication and cooperation between all team members is crucial for the safe administration of PHP treatments.
CLINICAL DEVELOPMENT PROGRAM
All of the U.S. clinical trials discussed in the following sections were all conducted with an older generation device (Gen 1). The (Gen 1) filter melphalan removal efficiency was consistent across the trials, with overall means ranging from 71.2% to 76.4%. A completely redesigned device (Gen 2) is currently approved (CE mark) and marketed in the European Union. It has been employed during compassionate use cases in the United States. The new filters have experimental in vitro and in vivo porcine model efficiencies of 98% or better for melphalan removal. Nearly 130 clinical procedures have been performed worldwide with the new Gen 2 device for treating various tumor metastases in the liver. Neither the Gen 1 nor Gen 2 device has FDA approval.
An open-label, phase 1, single-center, sequential, dose escalation study was used to determine the maximum tolerated dose of melphalan administered by PHP to patients with unresectable hepatic metastases from melanoma (cutaneous and ocular, n = 16) and assorted other tumors (n = 18). This study established a maximum tolerated dose of melphalan of 3.0 mg/kg ideal body weight (IBW).19 A phase 2, open-label, single-center, nonrandomized study was conducted (simultaneously with the phase 3 multicenter trial) to examine the efficacy of PHP in patients with unresectable primary hepatic malignancies and unresectable metastatic hepatic malignancies from other tumor types (GI, adenocarcinoma, neuroendocrine, and cutaneous or ocular melanoma). This study included four patients with melanoma who were not candidates for the phase 3 trial.
The Phase 3 Trial
The phase 3 study was a randomized, controlled, multicenter trial to evaluate the efficacy, safety, and tolerability of PHP treatment versus best alternative care (BAC) in patients with unresectable hepatic metastases from cutaneous or ocular melanoma. This study used the aforementioned Gen 1 device. The primary end point was hepatic progression-free survival. Notably, this was the first randomized, controlled, phase 3 clinical trial to include patients with ocular melanoma and was conducted following special protocol assessment review by the FDA.
In the phase 3 study, patients in the BAC group were allowed to cross over to PHP treatment at the time of documented hepatic progression provided they continued to meet the eligibility criteria for the study at the time of crossover. The crossover study design was agreed with FDA because of (1) the lack of any standard or effective therapy for metastatic melanoma with liver-dominant disease, particularly for ocular melanoma, and (2) it would be unethical to not allow for crossover to a potentially active and lifesaving therapy that had shown positive results in a phase 1 study. It was anticipated that the crossover design would confound analyses of survival. Phase 2 and phase 3 study patients are being followed for survival.
Study Population
This clinical trial was conducted in the United States at the National Cancer Institute and nine additional sites. The demographic and baseline characteristics of patients enrolled in the PHP clinical trials were representative of the target indication of unresectable metastatic melanoma in the liver. There were no statistically significant differences between the PHP (n = 44) and BAC (n = 49) groups for any demographic or baseline disease characteristic in the phase 3 study, with the exception of Eastern Cooperative Oncology Group (ECOG) performance status which was statistically significantly worse in the PHP group, with more ECOG status 1 patients, than in the BAC group. In all of the studies, most of the patients were white and younger than 65 years of age.
Most patients (n = 83) in the phase 3 study had ocular melanoma with five or more hepatic lesions at baseline. All 4 patients in the melanoma subset in the phase 2 study had ocular melanoma, and of the 16 patients in the phase 1, 12 had ocular melanoma and 4 had cutaneous melanoma. The median time since diagnosis of the primary tumor across the studies ranged between 38.1 months and 55.7 months, and the median time since diagnosis of hepatic metastasis ranged between 1.9 months and 6.5 months for the phase 3 study and the phase 1 study, respectively, indicating recent life-threatening disease relative to the overall length of disease; this was not evaluated for the phase 2 study. In the phase 3 study, patients who received PHP had a median of three cycles of treatment (range 1 to 6).
Efficacy
In all three studies, both hepatic and extrahepatic lesions were evaluated using the Response Evaluation Criteria In Solid Tumors (RECIST); however, only the presence or absence of extrahepatic lesions was recorded and there was not a fixed schedule for the evaluation of extrahepatic lesions. In the phase 3 study, the primary efficacy end point, hepatic progression-free survival (hPFS), assessed by the independent review committee (IRC), was met. Investigator assessments were consistent with the IRC. Alexander20 reported a median hPFS of 8.0 months for PHP compared to 1.6 months for BAC (HR = 0.35 [0.23−0.54]; P < .001) and an overall response rate of 32% for PHP versus 2% for BAC (P = .001). Fifty-five percent of patients had crossed over from BAC to PHP upon hepatic progression, and as anticipated, no significant difference in overall survival could be demonstrated.
A consistent treatment benefit was seen for PHP treatment across the primary and secondary efficacy end points in the phase 3 study. The exception was overall survival, which was confounded by the high number of BAC patients who crossed over to PHP treatment. To date, most patients still alive in the phase 3 study received PHP treatment (7/10 patients: 2 who were randomized to PHP and 5 who crossed over). Historically, only a rare patient with liver-dominant melanoma is a long-term survivor (approximately 2%). The results seen in the phase 3 study were reinforced by consistent results for hPFS and hepatic Overall Response (hOR) in the melanoma subset in the supportive phase 1 and phase 2 studies and may serve as surrogates for overall survival because the overwhelming cause of death in melanoma patients with liver-dominant disease is early liver progression and liver failure.
Safety
The safety profile of the melphalan/PHP system has been evaluated in three clinical studies in patients with unresectable hepatic metastases or primary liver tumors. A total of 155 patients received PHP treatment in these studies: 33 in the phase 1 study, 52 in the phase 2 study, and 70 in the phase 3 study (42 randomized to PHP and 28 who crossed over to PHP). Laboratory monitoring was conducted more frequently in the PHP group than in the BAC group.
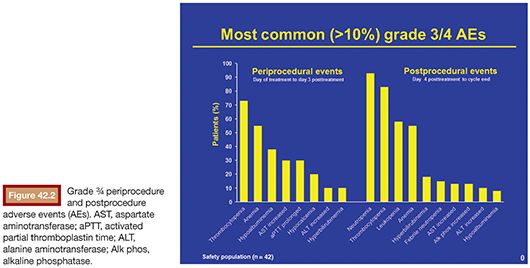
In all three clinical studies, adverse events were analyzed by two procedural periods: the periprocedure and postprocedure periods (Fig. 42.2). The periprocedure period was defined as the period from the date of the planned procedure until the earlier of 3 days or patient discharge from the hospital. Adverse events reported during this period are more likely to be device/procedure-related adverse events. The postprocedure period was defined as the time from the end of the periprocedure period until the day before the date of the next treatment cycle. For the last treatment cycle, the end of the postprocedure period was the earlier of either the date of death or 30 days after the date of the final study dose. Adverse events reported during this period are more likely to be melphalan-related adverse events.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree