Ricardo Yamada • Andre Uflacker • Austin Bourgeois • Joshua D. Adams • Marcelo Guimaraes
Onyx (Covidien, Irvine, California) was initially manufactured as a dialysis matrix for separating immunoglobulin from albumin and then as a matrix for controlled release of chemotherapeutics.1 However, it was also found to have embolic properties, and its clinical use for this purpose was first described in 1990 for embolization of intracranial arteriovenous malformation (AVM).2
At that time, Onyx showed promising results, overcoming the drawbacks of cyanoacrylate, a well-known embolic liquid agent commonly used for the same purpose. The lack of adhesiveness and slow copolymerization rate permit more distal nidus embolization, sometimes including the proximal venous outflow, without significant risk of microcatheter entrapment. After several studies, including a multicenter randomized trial comparing Onyx and cyanoacrylate, in July 2005, the U.S. Food and Drug Administration approved its use for intracranial AVM embolization.3 European device approval (CE marking) preceded that in the United States by approximately 5 years for embolization of AVMs and intracranial aneurysms as well. Since then, given the safety and effectiveness of Onyx in the intracranial vasculature, use on peripheral organs has been described and successfully applied. Nowadays, it has been used mainly for the treatment of peripheral AVMs and abdominal aorta stent graft–related endoleaks.4–7 In addition, Adamus et al.8 published a series of cases describing 23 patients in whom Onyx embolization was successfully performed, including treatment of the renal, hepatic, iliac, and bronchial arteries and esophageal varices. The authors concluded that Onyx offers advantages over other embolic agents due to good controllability and faster vessel occlusion.8
DEVICE/MATERIAL DESCRIPTION
Onyx is a liquid permanent embolic agent composed of ethylene vinyl alcohol (EVOH) copolymer dissolved in dimethyl sulfoxide (DMSO) and micronized tantalum powder. The latter provides contrast for fluoroscopic visualization. The nonadhesive and viscous properties make it a unique agent, mostly differing from the other two known liquid embolic agents—glue and dehydrated alcohol. Its nonadhesive characteristic significantly decreases the risk of microcatheter entrapment, compared to glue (Table 10.1). The higher viscosity allows controlled deployment, which is extremely difficult to achieve with dehydrated alcohol.
The Onyx package includes three 1-mL delivery syringes, two labeled for Onyx use (white plunger) and one for DMSO (yellow plunger); one 1.5-mL vial of Onyx; and one 1.5-mL vial of DMSO (Fig. 10.1). The Onyx white plunger syringe has lower friction compared to the DMSO yellow one, which gives better control while the agent is being injected. DMSO is a solvent and is used to wash out the microcatheter and prevent immediate direct contact between Onyx and the bloodstream, which ultimately triggers the solidification. Because of the theoretical risk of melting down non–DMSO-compatible microcatheters, it is recommended using only pretested microcatheters, including Marathon, Rebar, Echelon, and UltraFlow (Covidien, Irvine, California). They have been extensively tested regarding their compatibility with DMSO, which is outlined further in Chapter 12. In addition, their dead space volume is provided in the microcatheter package (dead space: volume necessary to fill up the lumen of the microcatheter completely).

Three different Onyx viscosities are available with different EVOH concentrations: Onyx 18 (with 6% of EVOH), Onyx 34 (with 8% of EVOH), and Onyx 500 (recommended for giant intracranial aneurysms). The second formulation is almost twice as viscous as the first one. Higher viscosity (Onyx 34) offers better control while the embolic agent is injected, but as the copolymerization process is faster, agent delivery in areas too distal to the microcatheter tip is more difficult. Therefore, this formulation is best suited for very high-flow lesion, with important vascular branches that must be preserved, and when the microcatheter tip is close or within the target lesion. On the other hand, a lower viscosity formulation (Onyx 18) is preferred when the microcatheter cannot be advanced any closer to the lesion and the target area is still too far away from the microcatheter tip. The lower viscosity and slower copolymerization time allow the liquid embolic agent to flow deeper, reaching more distal areas of the lesion. As one can note, it is usually a trade-off between a more controlled delivery but with less chance of full lesion embolization or complete lesion embolization with higher risk of occlusion of nontarget vessels.
Another unique Onyx characteristic is the “outside-in” solidification process. First, the outer layer forms a solid cast while a spongy or foamlike consistency is created inside, similar to what happens to the volcano lava when it cools off. The solidification process, also called copolymerization, starts once Onyx comes in contact with ionic fluids, such as blood, iodine contrast material, and saline solution, and usually takes 5 minutes to reach a solid and stable consistency. In contrast to dehydrated alcohol that causes an acute and severe endothelitis, Onyx induces a mild inflammatory reaction in the adjacent vessel walls, and as a nonabsorbable embolic agent, recanalization of the embolized area is nonexistent.9
CLINICAL APPLICATIONS IN PERIPHERAL EMBOLIZATIONS
Early report on peripheral Onyx application date back to 2001, when Martin and colleagues6 reported Onyx embolization of endoleak types II and Ia. All patients presented with decreased aneurysmal sac diameter after a mean follow-up of 19.2 weeks.6 Since then, multiple reports have been published, describing successful Onyx embolization for different pathologies among different peripheral vascular territories, including visceral aneurysm; pseudoaneurysm; hemoptysis; gastrointestinal bleeding; and abdominal, pulmonary, and upper and lower extremity AVMs.4,5,10–13 For all these entities, Onyx has been shown to be as useful as other conventional embolic agents or sometimes even more effective due to faster vessel occlusion and good delivery control.8 Moreover, Onyx can be considered the embolic agent of choice for peripheral AVMs and type II endoleak embolization.14
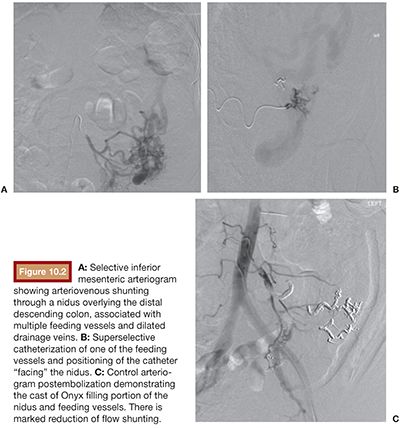
Typically, AVMs are challenging lesions to be treated due to the combination of potential high-flow environment and the need for distal intranidus embolization. As a liquid embolic agent, Onyx can reach small vessels deep within the nidus, sometimes far away from the delivery microcatheter. At the same time, different predictable viscosities allow safe agent delivery, with low risk of distal nontarget embolization (Fig. 10.2).
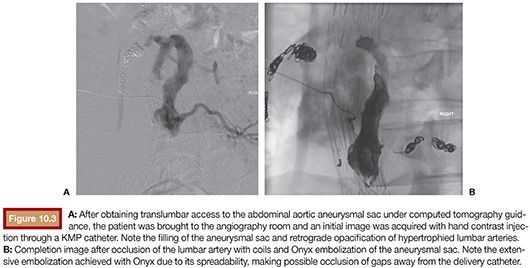
With a similar flow dynamics, type II endoleaks can behave like AVMs, having the aneurysmal sac as a functional “nidus,” associated with multiple inflow and outflow vessels. In this scenario, Onyx has been shown as an extremely efficient embolic agent, achieving complete filling of the open channels within the aneurysmal sac and also proximal occlusion of the involved vessels (Fig. 10.3).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








