Ryan M. Hickey • Robert J. Lewandowski • Riad Salem
R adioembolization refers to the transcatheter, intra-arterial injection of micrometer-sized embolic particles loaded with the high-dose radioisotope yttrium 90. Radioembolization combines the selective angiographic techniques essential to all embolization procedures with an understanding of radiation administration and safety, including radiation dosimetry and radiation dose modification based on tumor characteristics and the patient’s clinical profile.
Radioembolization delivers an internal radiation source and is therefore considered brachytherapy. Whereas the radiosensitive nature of normal liver tissue has limited the role of external beam radiation in the treatment of primary and metastatic hepatic malignancies, radioembolization allows for the safe administration of high and therapeutic doses of radiation. More specifically, the likelihood of developing severe radiation-induced liver disease may exceed 50% for external beam radiation doses greater than 35 to 40 Gy, whereas radiation doses greater than 150 Gy have been safely administered with radioembolization.1–4
The high tumoral radiation doses achieved with radioembolization rely on the selective intra-arterial injection of the radioactive particles and the preferential deposition of these particles within tumor as opposed to normal liver tissue due to differences in normal and tumor tissue perfusion. Whereas normal liver parenchyma receives most (75%) of its blood supply from the portal vein, hepatic malignancies, particularly hypervascular tumors such as hepatocellular carcinoma (HCC), derive most of their blood supply from the hepatic arteries.5,6
The radioisotope yttrium 90 is irreversibly incorporated into glass or resin microspheres that range in size from 20 to 30 µm (glass) or 20 to 60 µm (resin). Yttrium 90 is a pure beta emitter with a half-life of 64.2 hours and tissue penetration ranging from 2.5 to 11 mm.7–11 Glass microspheres (TheraSphere; Nordion, Kanata, Ontario, Canada) were approved in 1999 by the U.S. Food and Drug Administration (FDA) under a humanitarian device exemption (HDE) for the treatment of unresectable HCC.12 Resin microspheres (SIR-Spheres; SIRTeX Medical Limited, New South Wales, Australia) were granted full premarketing approval in 2002 by the FDA for the treatment of unresectable colorectal metastases in conjunction with intrahepatic floxuridine.13
PATIENT SELECTION
The patient selection process for yttrium 90 (Y90) radioembolization is multifactorial and involves an assessment of the patient’s burden of disease, biochemical profile, and performance status. Ideally, patients should have liver-only disease with a tumor burden comprising less than 70% of the liver volume. There should be adequate hepatic reserve, as indicated by the patient’s liver function tests with a bilirubin less than or equal to 2 mg/dL and albumin greater than 3 g/dL. The prothrombin time is a sensitive indicator of hepatic synthetic function and should correspond to a normal international normalized ratio (INR). Cancer-related symptoms should be minimal, corresponding to an Eastern Cooperative Oncology Group (ECOG) score of 0 to 2. Although the presence of portal vein thrombosis (PVT) has been traditionally considered a contraindication to hepatic arterial embolization procedures, radioembolization has been shown to be safe and effective in the setting of partial and branch PVT.14,15 Radioembolization should be performed with caution in patients who have had intervention or surgery involving the ampulla of Vater due to the risk of developing hepatic abscesses. These patients should receive aggressive antibiotic coverage at the time of the procedure that is continued into the postprocedural period.
TECHNIQUE
Y90 radioembolization is performed on an outpatient basis and involves two separate angiography procedures. An initial mapping angiography is performed, at which time a radioisotope lung shunt fraction is also determined. Y90 microspheres are then administered during a separate treatment angiography.
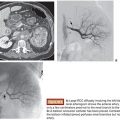
Due to the frequency of hepatic arterial variants and the propensity of hepatic tumors to exhibit arteriovenous shunting, the mapping angiography and lung shunt fraction calculation are integral to radioembolization. Meticulous visceral and hepatic angiography is required to establish the planned treatment volumes that will be used for radiation dosimetry as well as to identify extrahepatic arterial pathways that could result in devastating toxicities and complications from nontarget radioembolization.
HCC is associated with a relatively high incidence of direct arteriovenous shunts that bypass the tumor capillary bed.16 The administration of microspheres smaller than these shunts could therefore result in direct shunting of the radioactive microspheres to the lungs, which can cause radiation pneumonitis at sufficient doses.17
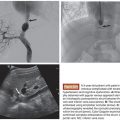
99mTc-macroaggregated albumin (MAA) is of similar size to the Y90 microspheres and expected to mirror their distribution, including in pulmonary shunting. 99mTc-MAA 2 to 4 mCi is administered via the proper, right, or left hepatic artery, depending on the planned treatment site, at the conclusion of the initial angiography. The patient is then transferred to nuclear medicine for acquisition of planar and/or single-photon emission computed tomography (SPECT) gamma camera images that are used to calculate the fraction of administered 99mTc-MAA activity to arrive in the lungs. It is worth mentioning that the time required to transfer patients from the angiography suite to nuclear medicine is an important factor, as the normal, time-dependent degradation of 99mTc-MAA over a prolonged period can result in a falsely increased calculation of lung shunting.18 For the most accurate calculation of lung shunting, it is recommended that the 99mTc-MAA be administered at the end of the mapping angiography and patients be transferred to nuclear medicine as soon as hemostasis has been achieved at the femoral access site. When lung shunting is identified, the cumulative pulmonary dose must be calculated along with the hepatic treatment doses. Pulmonary doses greater than 30 Gy per treatment or greater than 50 Gy cumulatively have been associated with the development of radiation pneumonitis.19
Hepatic arterial anatomy and corresponding treatment volumes are determined with the mapping angiography. Because the administered radiation dose depends on the activity of Y90 administered to a volume of tissue, common variants of hepatic vasculature can significantly alter the treatment plan. For example, in a patient with multinodular HCC limited to the right hepatic lobe, standard anatomy may allow for Y90 administration via the right hepatic artery. However, in the presence of an accessory hepatic artery, which typically perfuses the posterior segments 6 and 7, one injection must be performed via the accessory right hepatic artery according to the volume of segments 6 and 7 while a separate injection must be delivered via the right hepatic artery according to the volume of segments 5 and 8. The target lobar and/or segmental volumes corresponding to the angiographic anatomy are measured from the patient’s preceding CT or magnetic resonance imaging (MRI) on a three-dimensional (3-D) workstation.
In addition to treatment planning, the mapping angiography provides a critical opportunity to identify extrahepatic arterial flow, particularly to the gastrointestinal tract, that could result in nontarget radioembolization. These vessels can either be eliminated with coil embolization during the mapping angiography or avoided by alterations in the treatment plan. A left gastric artery may have to be embolized to avoid reflux of Y90 to the stomach during treatment via a replaced or accessory left hepatic artery. The gastroduodenal and right gastric arteries are often prophylactically embolized, particularly when using the more embolic resin microspheres. The relative location of the cystic, supraduodenal, retroduodenal, falciform, and phrenic arteries should also be considered in determining the site of Y90 injection. An in-depth review of angiographic considerations and relevant variant anatomy has been previously published.20
A simplified matrix has been described for the treatment planning of patients with unresectable HCC based on the extent of disease and the patient’s total bilirubin level.18 For patients with uninodular disease and a normal bilirubin, Y90 can be administered via a lobar or segmental injection. For patients with uninodular disease but elevated bilirubin, treatment should only proceed if a segmental perfusing vessel can be isolated, resulting in high-dose radiation to the tumor and minimal distribution to the normal liver parenchyma. For patients with multinodular and/or bilobar disease and a normal bilirubin level, staged lobar treatments may be performed. In the setting of abnormal bilirubin and multinodular/bilobar disease, the risk of Y90 treatment may be unacceptably high.
DOSIMETRY
Y90 radiation dosimetry uses volumetric calculations of liver tissue (glass microspheres) or tumor burden and body surface area (resin microspheres). Several different software packages are available to calculate 3-D tissue volumes from triphasic CT or multiphasic contrast-enhanced MRI. Volumetric calculation and treatment planning require a sound understanding of the Couinaud hepatic segments and their anatomic landmarks on cross-sectional imaging.
For glass microsphere (TheraSphere) dosimetry, the volume that is measured and used in the dosimetry calculation is the volume of liver tissue that is perfused by the vessel infused. In other words, it is the volume of the liver segments being perfused by the vessel of interest.
Glass microspheres (TheraSphere) are available in vials of several different activities that are dispensed weekly on Wednesday by the manufacturer (Nordion) and calibrated at 12:00 noon (Eastern Standard Time) of the following Sunday. The microspheres have an approximate activity of 2,500 Bq per sphere.1
The recommended activity that should be administered to a tumor-containing hepatic lobe should correspond to a dose between 80 and 150 Gy. Patients with significant cirrhosis should be treated conservatively with doses between 80 and 100 Gy, whereas patients without cirrhosis may be treated at higher doses between 100 and 150 Gy. The most commonly used dose range at our institution is between 100 and 120 Gy. Activity required to deliver the desired dose can be calculated according to the equation:
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree