Fig. 11.1
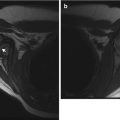
Cartilage between the femoral head and greater trochanter is continuous in this 3-months-old, as they develop from one block of cartilage (coronal US). Ilium (arrow), triradiate cartilage (open arrowhead), femoral head (asterisk), greater tuberosity (crooked arrow), and metaphysis (white arrowhead). Note the specular foci of echogenicity in the femoral head and greater tuberosity, due to blood vessels viewed in cross section. S superior, L lateral
The medial and lateral femoral circumflex branches of the common or deep femoral arteries supply the developing femoral head and neck; the femoral ligament artery is a branch of the obturator artery but is inconstant. The pattern of vascular supply to the head is thought to change during the first 3 years of age such that a few discrete branches of the medial circumflex artery supply the epiphysis, and the lateral circumflex artery supplies the greater trochanter region. The femoral and gluteal veins drain the femoral head and neck [4].
At birth, the ossific pattern of the femoral neck is a meshwork of uniformly distributed fine trabeculae. The adult pattern develops slowly; in one half of children between 12 and 18 months, the principal medial and lateral arching pattern is faintly evident [5]. These linear condensations are well developed by age 5; trabeculae aligned with the medial pattern are eventually seen as extensions on the epiphyseal side of the growth plate adjacent to the ossification center. By adolescence, short secondary medial and lateral arches develop inferior to the major lines of force. These trabeculae, with a large surface area-to-volume ratio, are more sensitive than cortical bone to systemic (endocrine, genetic, metabolic, nutritional, toxic) or local (trauma, infection) factors [6].
Both the timing and the symmetry of ossification of the femoral head vary. As with many structures, the proximal femoral epiphysis ossifies earlier in girls (2–6 months versus 3–7 months in boys). Ossification is seen in the femoral head in one half of all infants by 4 months and in more than 90 % by 7 months (200 days) [7]. With so broad a normal range, great variability is possible, and one must be cautious about labeling absent or decreased ossification as pathologic.
During infancy, the ossific center usually mineralizes and grows uniformly. However, it may appear crenulated, stippled, or granular, and multiple separate centers may be seen. These typically become homogeneous by mid-childhood, but occasionally a granular appearance or even a vertical cleft may persist. The medial portion of the epiphysis may appear relatively dense due to the superimposed lower acetabular wall.
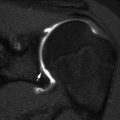
Discrete notches at the vertex are common; these represent ossific defects only, since the contour of the epiphyseal cartilage over the notch is smooth [8]. This defect should not be confused with the fovea centralis at the insertion of the ligamentum teres, located in the medial aspect of the epiphysis, just above the femoral head equator (Fig. 11.2).
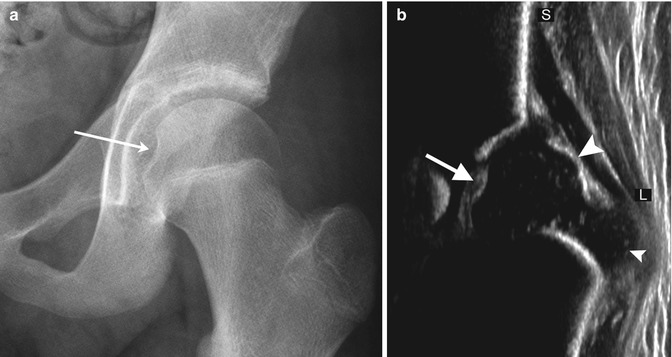

Fig. 11.2
Normal fovea (arrow) on (a) radiograph and (b) coronal neutral US. S superior, L lateral. The small arrowhead indicates the greater tuberosity, and the large arrowhead indicates the femoral head
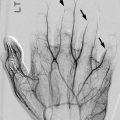
Multiple ossification centers are infrequently seen at the greater trochanter (Fig. 11.3). The absence of symptoms distinguishes these normal variants from bursitis with calcific fragments or a “tug” lesion.
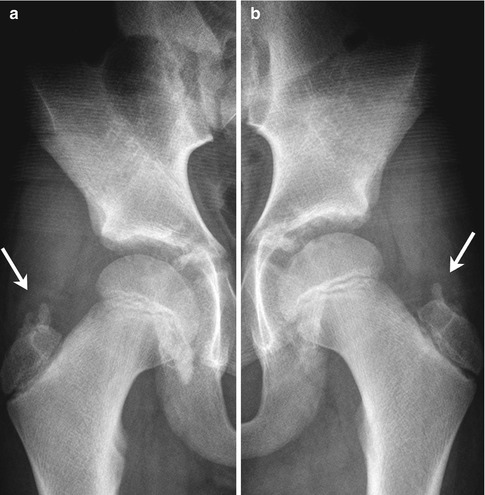

Fig. 11.3
(a, b) Normal irregular ossification centers (arrows) of greater trochanters in an asymptomatic 10-year-old boy
1.2 Pelvis
Variations in contour and symmetry are frequent in the pelvis, as they are elsewhere. Numerous apophyses contribute to formation of the pelvis, and the timing of their appearance and fusion varies, as does their appearance during ossification. Some can be confused with avulsion fractures and even with tumors. The more common radiographic features are discussed below.
A secondary ossification center forms in the anterior superior iliac spine at age 13–15, fusing to the ilium by age 16–18. It is usually seen well only in slightly oblique projections. Although avulsion of this center may occur during vigorous activity, it may normally be separated from the body of the ilium by a substantial layer of cartilage. As at other apophyses, the diagnosis of traumatic separation should be made only if there is adequate clinical correlation. Ossification centers are also present at the iliac crest and at the anterior inferior iliac spine.
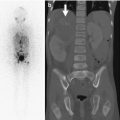
The timing of closure of the ischiopubic synchondrosis is extremely variable, closing as early as 3 years of age, but incomplete in 18 % of children at age 12. The lucent cartilaginous component often enlarges before closure and may appear swollen or bubbly for up to 3 years (Fig. 11.4). Occasionally, there is cyclic swelling, bone union, reopening, swelling, and repeat closure, sometimes associated with pain. Unilateral synchondrosis expansion can be found in 57 % of children at age 7 [9]. In almost one half of patients, scintigraphy reveals bilateral but unequal uptake in this area. This asymmetry is well defined, does not extend into adjacent bone, and is never more active than that adjacent to the triradiate cartilage (Fig. 11.5). Magnetic resonance imaging (MRI) can be misleading, as increased asymmetric signal makes distinguishing between normal variation and pathology difficult [10]. Clinical correlation is extremely important, as young athletes often experience muscle strain in this region, and this may not necessarily correlate with radiographic asymmetry (see Chap. 12).
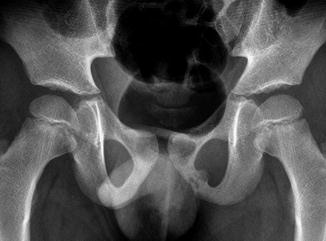
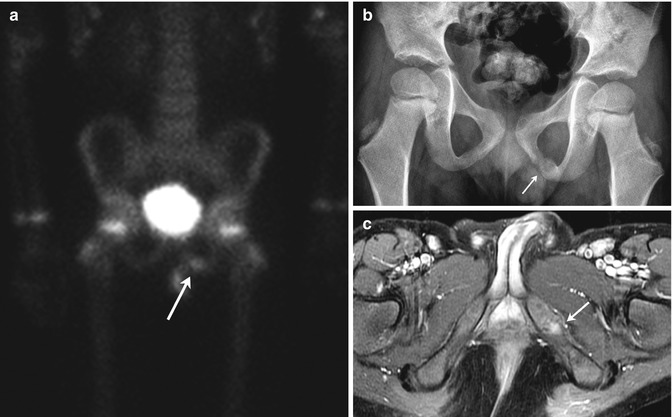

Fig. 11.4
Normal asymmetric closure of the ischiopubic synchondrosis in a 7-year-old boy. The right has closed, but the left is bubbly and expanded

Fig. 11.5
Normal asymmetry in the ischiopubic synchondrosis in a 5-year-old boy with an adrenal tumor. (a) Asymmetric increased uptake at the left ischiopubic synchondrosis (arrow) is less intense than at the physeal cartilage. (b) Expansion, sclerosis, and lucency (arrow) on the left. (c) T1-weighted (T1-W) fat-suppressed (FS) gadolinium-enhanced (GE) MRI shows mildly increased signal intensity (arrow) but no mass
The appearance of the pubic bones varies. The pubic symphysis is not always perfectly symmetric, and variant ossification centers infrequently develop in this area (Fig. 11.6). Vertical clefts and multiple centers of ossification are occasionally noted in the superior pubis at birth [11, 12]. During adolescence, accessory ossification centers may be seen in or just below the pubic symphysis. Irregularity of the lateral borders of the ischium may be quite striking in mid-childhood just before secondary ossification centers appear.
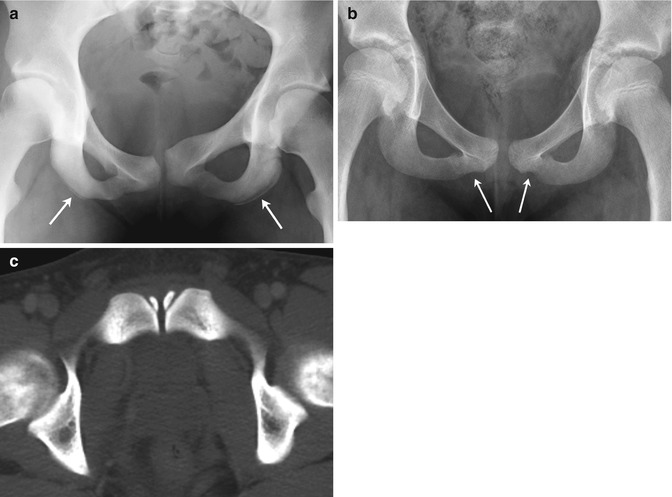

Fig. 11.6
Normal variation of the pubic bones. (a) Asymmetric ischial ossification centers (arrows), along with asymmetric pubic bones and symphysis. (b) Undulating margins of the inferior pubic rami (arrows) (there was no history of trauma). (c) Normal ossification centers adjacent to the symphysis in a 17-year-old boy (axial CT)
The os acetabuli is a well-demarcated ossicle just below the anterior-inferior iliac spine, at the most lateral margin of the acetabular cartilage rim. Uncommonly seen, this structure ossifies as a discrete triangular body between the ages of 11 and 20 (Fig. 11.7). Usually fusing to the acetabular margin by age 30, it may remain unfused through adulthood, when it is sometimes mistaken for an avulsion fracture. A similar ossification center may be present at the roof of the acetabulum.
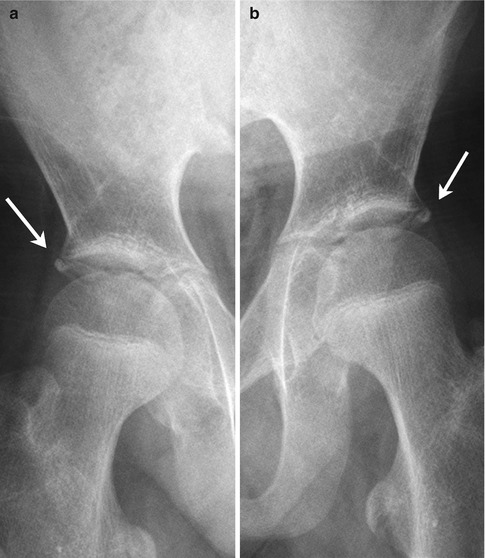

Fig. 11.7
(a, b) Bilateral os acetabuli (arrows) in a 14-year-old boy
Variant ossification at the acetabulum can cause confusion. A well-defined but wavy outline of the superior posterior acetabular margin is common. Sharp beaking of the medial intrapelvic portion of the acetabular triradiate synchondrosis is often prominent between ages 10 and 12 (Fig. 11.8). This is associated with inward bulging of the medial acetabular wall, producing a transitory relative acetabular protrusion that disappears by the end of puberty. Although rarely apparent on radiographs, multiple bone fragments are apparent on computed tomography (CT) in and around the triradiate cartilage in the adolescent prior to growth plate closure (Fig. 11.9).
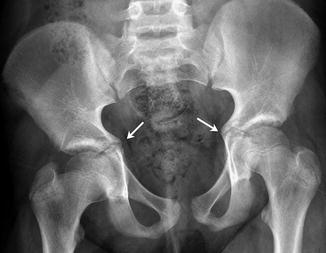
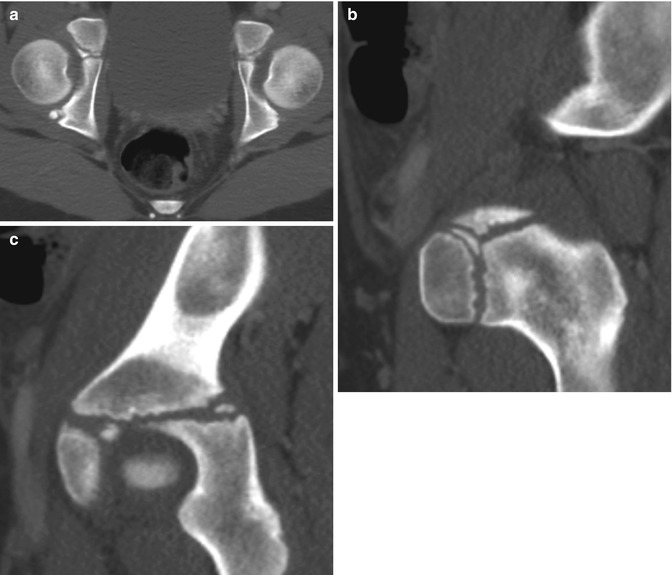

Fig. 11.8
Normal bilateral beaking and medial protrusion of the triradiate cartilage (arrows) in an 11-year-old boy

Fig. 11.9
Multiple normal ossification centers in the triradiate cartilage in a 10-year-old girl. Axial (a) and sagittal reformatted (b, c) CTs
2 Proximal Focal Femoral Deficiency (PFFD)
PFFD is a rare, nonhereditary defect of the femur, defined by defective development of the proximal femur and iliofemoral articulation, limb malrotation, and leg length discrepancy. The lower extremity typically appears short and thick, held in abduction, flexion, and external rotation [13]. It ranges in severity from mild dysgenesis of the proximal femur (Fig. 11.10) to almost complete absence of the femur, with only a small distal stub (Fig. 11.11). The defect is usually isolated and unilateral, although it may be a component of the caudal regression syndrome in infants of diabetic mothers. Incidence is about 1 in 52,000 births [13]. Total femoral agenesis, phocomelia, simple leg-length discrepancy without a proximal focal abnormality, and congenital femoral neck varus without shortening are not included in the definition of PFFD.
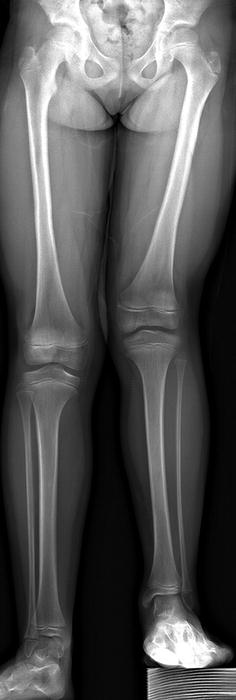
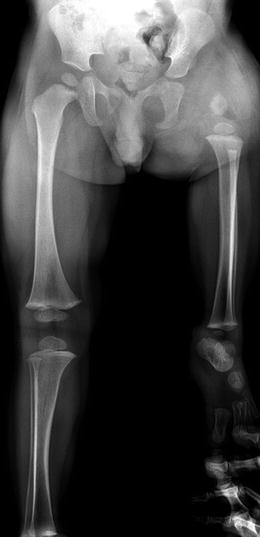

Fig. 11.10
Very mild PFFD. The left femur is short, but the acetabulum and femoral head/neck are well formed. The left fibula is mildly hypoplastic but the tibia essentially normal. This is Pappas Class IX, which is essentially a congenitally short femur. The Aitken system does not classify this appearance

Fig. 11.11
Aitken Class D PFFD (Pappas Class 2). The femoral fragment is very small, and there is no ossified femoral head. The acetabulum has not formed. Note the thick soft tissues about the hip. The fibula is hypoplastic
Associated anomalies of the distal bones and musculoligamentous structures as well as secondary effects on articulating structures at the hip and knee are common. Tibial and especially fibular hypoplasia can occur. Femoral shortening averages 60 %; tibial and fibular length discrepancies average 7 % and 28 %, respectively. Accurate prediction of ultimate discrepancy is possible due to the linear nature of growth impairment [14] (see Chap. 2).
Treatment aims to maximize function and stability and varies depending on the severity of the defect. For milder PFFD, treatment may consist solely of femoral lengthening. In more severe forms, amputation and prostheses may be required (Fig. 11.12). Van Ness rotationplasty—fusion of the distal tibia to the short femur with 180° rotation of the tibia—is used to gain flexion at the rotated ankle joint and prepare a stump for prosthesis, essentially transforming the ankle into a knee [15].
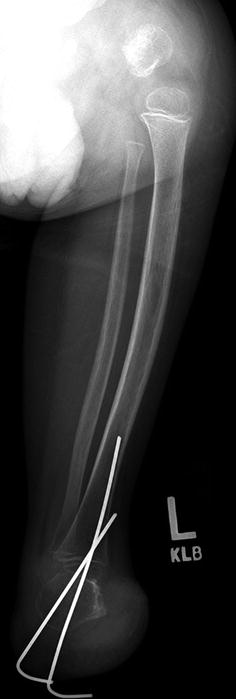

Fig. 11.12
PFFD treated with partial amputation of the foot and fusion of the ankle joint (same patient as Fig. 11.11)
Imaging
While PFFD may be difficult to classify at an early stage, doing so improves prognosis by helping to direct appropriate therapy. Of the many classification systems available, the Aitken and the Amstutz [16] are often employed. The Aitken system (Fig. 11.13) (Table 11.1) divides PFFD into four classes [17]. In class A, the femoral head is well formed and attached to a short but otherwise well-formed femur; the acetabulum is normal. A bony connection eventually forms between the proximal femur and the shaft, but often with a pseudarthrosis. In class B, the femoral head is also present, but there is no bony connection between it and the femoral shaft at maturity; there may be no cartilaginous connection either. The acetabulum is either adequate or moderately dysplastic. Class C is defined by complete absence of ossification of a femoral head. A short femur tapers proximally and has a small proximal tuft. The acetabulum is severely dysplastic. Both the femoral head and the acetabulum are absent in class D, and the femoral segment is even shorter and more deformed, often pointed proximally. The Amstutz system further divides Aitken A into types 1 and 2, based on characteristics of the femoral neck (cartilaginous in type 1; pseudarthrosis in type 2); Aitken B through D are termed Amstutz 3–5. These systems predict femoral head development and its relation to the acetabulum and femoral neck. However, prediction of the final relationships is not always possible. More complex systems are also available (Fig. 11.14).
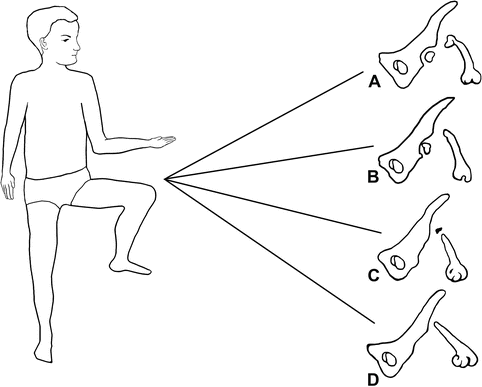
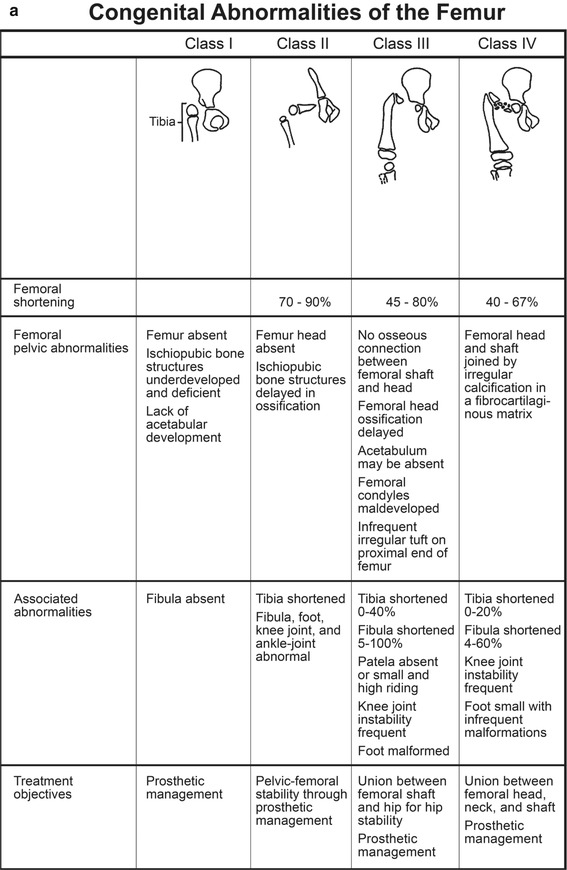
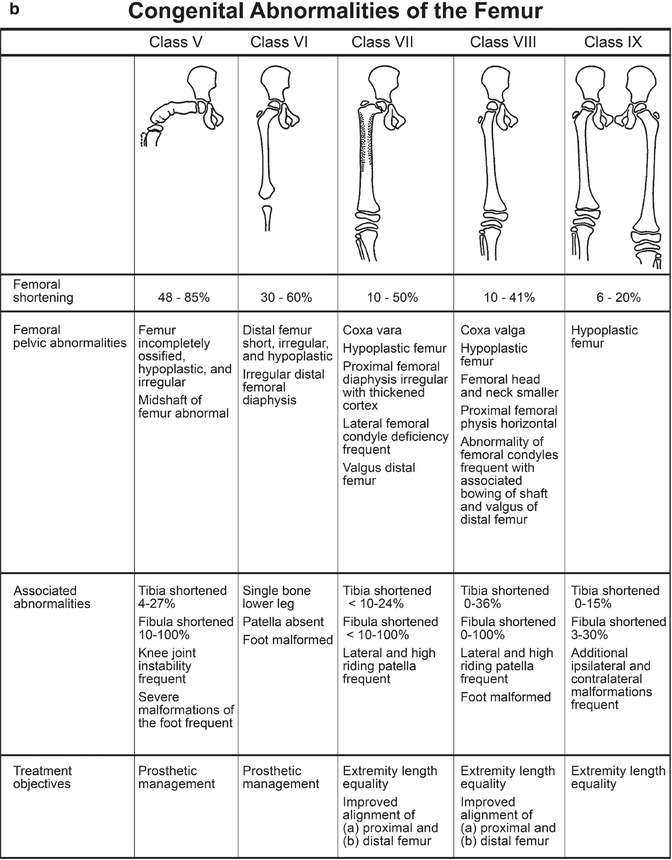

Fig. 11.13
The four categories of the Aitken PFFD classification system
Class | Femoral head | Acetabulum | Femoral segment | Relationship among components of femur and acetabulum at skeletal maturity |
|---|---|---|---|---|
A | Present | Normal | Short | Bony connection between components of femur |
Femoral head in acetabulum | ||||
Subtrochanteric varus, often with pseudarthrosis | ||||
B | Present | Adequate or moderately dysplastic | Short, usually proximal bony tuft | No osseous connection between head and shaft |
Femoral head in acetabulum | ||||
C | Absent or represented by ossicle | Severely dysplastic | Short, usually proximally tapered | Possible osseous connection between shaft and proximal ossicle |
No articular relation between femur and acetabulum | ||||
D | Absent | Absent | Short, deformed | None |
Obturator foramen enlarged | ||||
Pelvis squared in bilateral cases |


Fig. 11.14
Ultrasound (US) offers a relatively simple initial evaluation of femoral head and acetabular development prior to ossification (Fig. 11.15) [19]. However, severe coxa vara can limit the sonographic window. MRI (Fig. 11.16) provides more detailed evaluation of non-ossified structures, and this can help the orthopedic surgeon determine treatment. It can determine whether a cartilaginous femoral head is present and characterize growth plate abnormalities. It also evaluates acetabular contour and the presence of an osseous, cartilaginous, or fibrous connection between the proximal and distal femur. It characterizes ligaments and muscles and if extended to include the knee, can identify the accompanying ligamentous abnormalities that are common there. All this information assists in determining surgical treatment [13, 20]. Intraoperative arthrography may also be used to assess hip mobility and guide treatment.
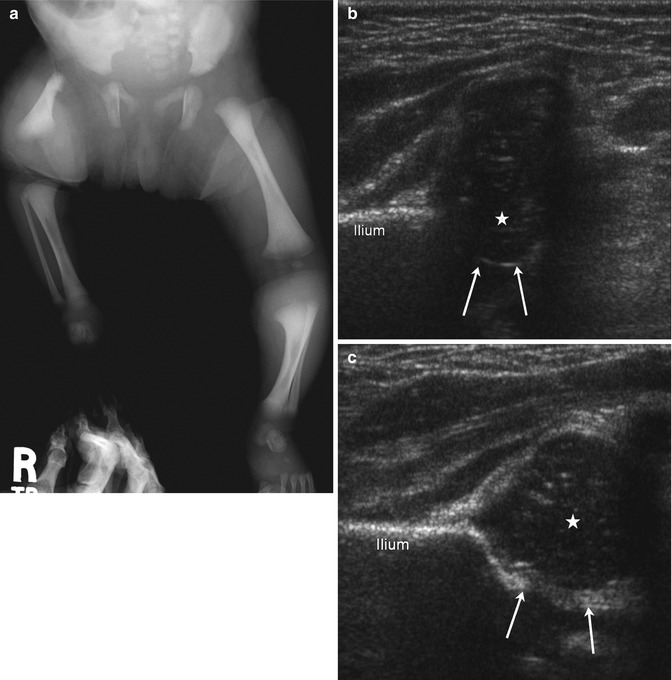
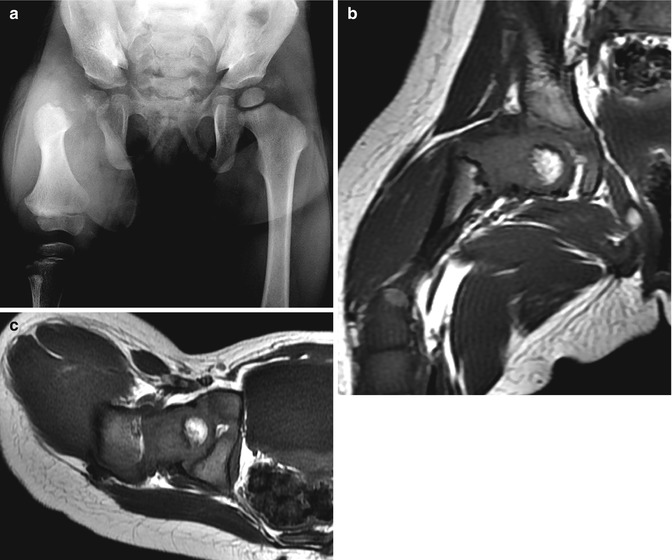

Fig. 11.15
Aitken Class A PFFD (Pappas Class 3) in an infant. (a) The right femur is short. Although the distal contour is fairly well formed, the proximal femur is hypoplastic, with a pointed upper end. Note coxa vara. The acetabulum is well formed, suggesting the presence of a femoral head. The tibia and fibula are small. (b) Coxa vara on coronal flexion US. The femoral head is small but well positioned. The acetabulum (arrows) is small. Hypoechoic cartilage lateral to the femoral head indicates varus configuration of the femoral neck. (c) Normal contralateral hip with the same notations

Fig. 11.16
Aitken Class B PFFD (Pappas Class 4). (a) Hypoplastic femur with ossification defect in femoral neck. T1-W coronal (b) and axial (c) MRIs show satisfactory positioning of femoral head and focal bone defect
Differential Diagnosis
Congenital short femur is the mildest form of PFFD (see Chap. 13). The proximal femur in developmental coxa vara appears similar to the milder forms of PFFD but lacks bone shortening. Dysplasias with proximal shortening as well as those with delayed proximal femoral ossification are in the differential.
3 Intrauterine Hip Dislocation
When associated with other congenital abnormalities, prenatal hip dislocation is considered teratologic, with multiple anomalies resulting from a common insult. Children born with hip dislocation along with, for example, arthrogryposis (Fig. 11.17) or cranio-carpal-tarsal dysplasia have primary teratologic dislocations. However, use of this term should be restricted to these complex situations, as simple prenatal dislocations can also occur. In either case, by the time of birth, prenatal dislocations are often firmly fixed by articular adhesions and secondary adaptive musculoligamentous changes and thus are difficult to reduce. Avascular necrosis (AVN) is a frequent treatment complication [21]. In general, intrauterine hip dislocation is unlikely to resolve completely without complications.
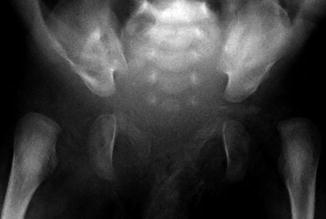

Fig. 11.17
Bilateral hip dislocation in a 1-month-old with arthrogryposis. Note steep obliquity of the exceedingly shallow acetabula. The left proximal femur is small
Prenatal dislocation can also result from paralytic states, such as myelomeningocele and other neuromuscular disorders. Many of these cases represent intrauterine postural deformity rather than true malformations. Flexion contracture of the knees and severe rigid varus and valgus deformity of the hindfoot may be present as well. This condition is associated with multiple gestations, breech presentation, and oligohydramnios.
4 Developmental Dysplasia of the Hip (DDH) (Box 11.1)
Box 11.1: Developmental Dysplasia of the Hip
Exam maneuvers | |
Barlow: detects displacement by pistoning flexed hip | |
Ortolani: attempts to reduce dislocated hip by flexion and abduction | |
Ultrasound | |
Dynamic examination | |
Coronal | Acetabular depth |
Labral configuration | |
Femoral head position | |
Transverse | Position of femoral head (resting and stressed) |
Static examination | |
Alpha angle | >/= 60° normal |
Beta angle | </= 55° normal |
This relatively common condition arises during the perinatal period and refers to the musculoligamentous, capsular, cartilaginous, and osseous changes that result from subluxation or dislocation of the femoral head. Although most dislocations occur during the perinatal period, dislocations do occur up to 1 year of age, often in patients documented to be normal before this time. Initial dislocation in later childhood is rare and generally results from abnormal muscular forces, trauma, or effusion.
In newborns, some degree of instability occurs in approximately 55 per 1,000 births, but most cases resolve within the first month without treatment, and the incidence of instability after that time is closer to 5 per 1,000 births [22]. The etiology and pathogenesis of infantile hip instability and displacement are subject to debate, but the origin of most dislocations seems to be multifactorial. Breech positioning and family history of joint laxity predispose to DDH, but many patients have none of these risk factors. Gender plays a role in this condition, which is four to eight times more common in female than in male infants.
Abnormal laxity is important in the development of femoral-acetabular incompetence. However, the degree of laxity that is considered abnormal is open to debate. In the first few days after birth, an ultrasound performed with stress can reveal as much as 6 mm of posterolateral displacement of the femoral head, but this usually resolves within a few weeks [23]. A hormonal contribution to ligamentous laxity has been suggested [24, 25].
The common intrauterine position of hip flexion plays an important role in maintaining proper position of the femoral head, and this in turn allows normal acetabular development. The hyperflexion of breech positioning leads to shortening and contraction of the iliopsoas muscle, predisposing to hip dislocation. Infants that have developed in the breech position are six times more likely to have DDH. In addition, the position of the fetus relative to the uterus and of one leg to the other probably account for what some studies have found to be increased involvement of the left hip.
Almost two thirds of infants with hip instability are firstborn. The maternal uterus and abdominal wall in a first pregnancy likely confine the infant, preventing the full intrauterine motion that is needed for normal femoroacetabular development. Associated conditions such as congenital torticollis [26] and congenital foot deformities such as metatarsus adductus and calcaneovalgus [27, 28] are also attributed to this “packaging” problem.
Although many patients with DDH have no affected family members, genetic factors play a role in hip dislocation [29]. If a parent has DDH, the risk to the child is 12 %. The risk to a younger sibling of a child with DDH and normal parents is 6 %. If one parent and one child are affected, a second child’s risk is 36 % [30]. In addition, DDH is more common in Caucasians than in those of African descent, and it is more common in Eastern Europe than in Africa and Asia. Infants who are swaddled with their legs in extension have increased risk of DDH; this is a common practice in certain populations, including some Native American cultures.
The significance of anatomic variation in the acetabulum or femoral neck is not settled, but the acetabulum tends to become shallower and the femoral head more hemispheric in late fetal life, perhaps promoting dislocation under appropriate conditions (e.g., breech positioning). The parents of children with DDH have been shown to have less acetabular coverage, suggesting that anatomic factors, possibly heritable, may be significant.
DDH is diagnosed by Barlow and Ortolani maneuvers, which are performed routinely on infants in the first few months of life. The Barlow maneuver pistons the flexed hip to detect displacement; the Ortolani maneuver assesses the dislocated hip with flexion and abduction to determine if it is reducible. Limited or asymmetric abduction of the hip is another sign of DDH and typically occurs after 2–3 months of age as the hip capsule tightens. Asymmetric thigh folds are neither sensitive nor specific for DDH. An audible clunk may occur as the hip dislocates or reduces, but clicks may be heard in normal hips. Leg-length discrepancy and limping are late signs.
When the clinical exam is abnormal or if there is a risk factor for DDH, US is performed to diagnose and determine the severity of the condition. The range of abnormalities found by US is extensive. The hip can be lax, mildly displaced with stress, dislocatable with stress, or dislocated at rest. In the latter case, the hip is positioned completely outside the acetabulum and may or may not be reducible. Laxity typically resolves within the first month. Subluxation may also resolve without treatment but must be monitored either clinically or by US. Dislocation is often treated at diagnosis with a flexion/abduction brace. Unsuccessful treatment usually necessitates intraoperative closed reduction and subsequent immobilization.
Clinical screening of all newborns by a skilled examiner has led to earlier diagnosis and better prognosis. Despite this, late diagnosis does occur in infants who were normal during the immediate postnatal period [31, 32]. It is possible that multiple examinations increase the instability of the hip [33]. In order to avoid late diagnosis, there has been considerable interest in sonographic screening. Some countries have instituted universal sonographic screening, whereas others only screen high-risk infants and those with a positive physical examination. The former approach is costly and some believe results in overdiagnosis and unnecessary treatment [34]. The more limited approach fails to diagnose DDH in infants who lack risk factors [35].
The American Academy of Pediatrics has recommended that all infants be screened by physical examination. If at any time there are positive physical findings (positive Ortolani or Barlow test), the patient should be referred to an orthopedist. If the physical examination is inconclusive, the infant should be reexamined in 2 weeks. If the examination is still inconclusive, orthopedic consult or US is recommended. Breech babies and female babies with a positive family history should have hip US at 6 weeks of age or a radiograph of the pelvis and hips at 4 months even if the physical exam is negative. Infants without risk factors or initial positive physical exam should be clinically monitored [36].
4.1 Imaging to Diagnose DDH
US of the hips has become the imaging standard in infants up to 6 months of age. The cartilage of the femoral head and acetabulum is readily depicted, whereas it cannot be seen on radiographs in young infants [37]. Hip US also benefits from real-time evaluation of hip motion.
The cartilaginous femoral head is recognized as a hypoechoic rounded structure with specular internal echoes that allow transmission of the sound wave to the acetabulum and the triradiate cartilage. Before it is visible radiographically, a small focus of echogenicity representing the nidus for the ossification center can be seen in most infants [38]. Like the femoral head, the hyaline cartilage of the acetabulum and the labrum is hypoechoic, whereas the fibrocartilaginous tip of the labrum is hyperechoic. The bony components of the acetabulum are brightly echogenic and prevent sound transmission. The joint space is clearly identified with movement of the femoral head and sometimes made more conspicuous by the presence of gas bubbles induced by examination maneuvers. The relationship of the femoral head to the acetabulum, the depth and configuration of the latter, and the position and morphology of the labrum are easily shown. Ultrasound can thus diagnose instability and cartilage abnormalities that are clinically and radiographically occult, and this has led to earlier diagnosis and treatment of DDH. Specular echogenic structures in the femoral head are caused by microvasculature. Doppler interrogation can demonstrate these vessels as curvilinear echogenic lines. However, this is primarily a research tool and not part of the routine exam [39, 40].
There are two popular methods of US examination, the dynamic and the Graf static technique. The dynamic technique [37, 38] emphasizes hip position and stability, with stress maneuvers that simulate Barlow and Ortolani maneuvers, whereas the static method is based on morphology as a reflection of hip position and stability [41]. Either method is suitable when used correctly. The American College of Radiology has established practice guidelines that recommend that both hips be examined in two orthogonal planes, including a coronal view at rest and a transverse view with and without stress [42].
Dynamic Hip Ultrasound
Standardized coronal and transverse images are obtained with hips in neutral and flexed positions. Scanning is performed over the lateral aspect of the hip joint with the patient supine or in shallow decubitus position, using a high-resolution linear transducer. The scan may be performed by a physician or technologist and with static images or stored clips. Experience is required to obtain diagnostic images. During treatment with an abduction brace such as a Pavlik harness, only coronal and transverse flexion views are performed. The harness should be removed and stress applied only at the request of the orthopedic surgeon. Ultrasound monitors improvement and helps guide adjustment of the device.
Coronal Views
The coronal views, performed with a neutral (Fig. 11.18) and with a flexed (Fig. 11.19) hip, show the femoral head and acetabulum as they appear on a frontal radiograph. Acetabular coverage of the femoral head should be 50 % or more. The normal osseous acetabulum should have a concave surface, and there should be a well-defined superolateral acetabular lip or margin. The labrum should resemble a sickle extending laterally over the femoral head with an echogenic tip at the outer margin.
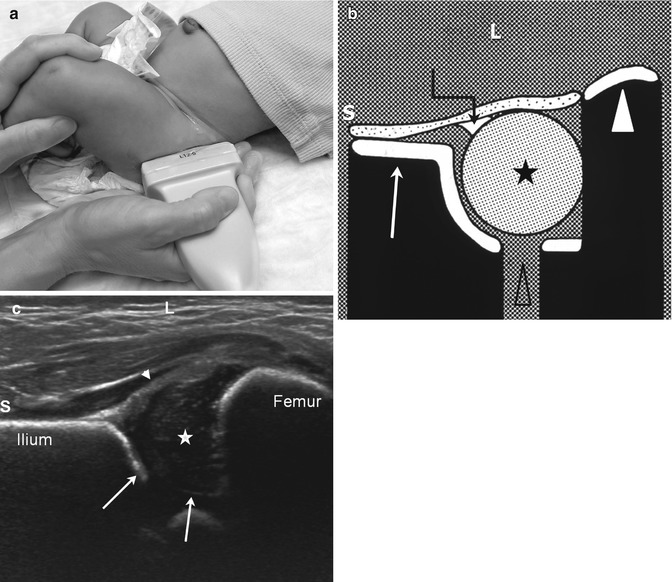
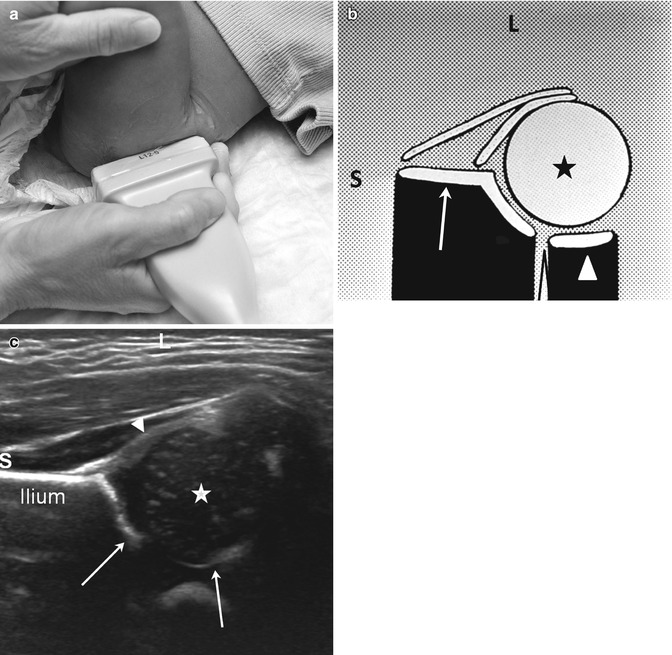

Fig. 11.18
Normal coronal neutral hip US. (a) The leg is in a physiologic neutral position (relaxed) with the transducer in the coronal plane. The Graf view is also obtained in this plane, but with the patient in the decubitus position and immobilized in a holder. (b) Diagram shows ilium (arrow), labrum (crooked arrow), femoral head (asterisk), triradiate cartilage (open arrowhead), and femoral shaft (white arrowhead). (c) Coronal neutral US shows the femoral head (star) positioned deep in the acetabulum (arrows) with no echoes between the femoral head and the medial acetabular wall. The iliac bone forms an echogenic structure superiorly, the femur, inferiorly. The labrum (arrowhead) curves over the femoral head. S superior, L lateral

Fig. 11.19
Normal coronal flexion view. (a) The hip is flexed to approximately 90° with the transducer in the coronal plane. (b) Diagram shows the ilium (arrow), femoral head (asterisk), and ischium (white arrowhead). (c) The metaphysis and shaft are not visible. The femoral head (star) is well positioned within the acetabular fossa (arrows) without intervening echoes. S superior, L lateral
Transverse Views
Performed posterolaterally in the axial plane, these are obtained according to the Harcke technique in both neutral (Fig. 11.20) and flexed (Fig. 11.21) positions. In the neutral position, the hypoechoic femoral head rests on the ischium posteriorly and on the pubic bone anteriorly, with the triradiate cartilage between these two echogenic structures. Aside from a thin layer of intervening hyaline cartilage, the femoral head should be positioned against these bones, with no intervening soft tissue. When flexed, the femoral head rests on the ischium with the triradiate cartilage and pubic bone seen medially and anteriorly, forming the acetabulum.
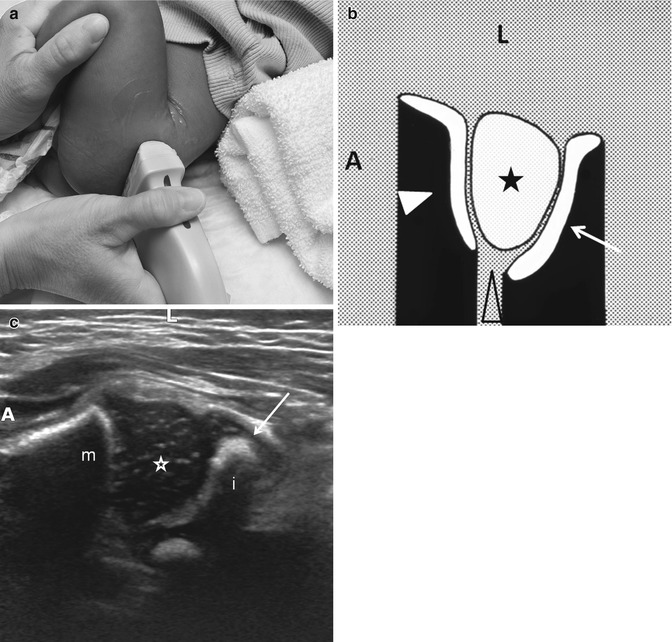
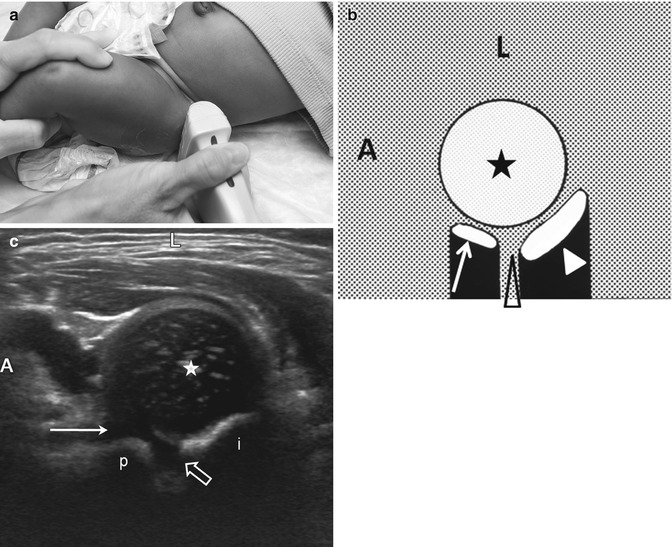

Fig. 11.20
Normal transverse (axial) flexion US. (a) The hip is flexed and the transducer rotated approximately 90°, parallel to the femoral shaft, posterolateral to the hip. (b) Diagram shows femoral metaphysis (white arrowhead), femoral head (asterisk), triradiate cartilage (open arrowhead), and acetabulum (arrow). (c) US shows the femoral head (star) with the metaphysis anterior (m) and the ischium (i) posterior without significant echoes deep in the acetabulum. The arrow indicates the posterior lip of the acetabulum. A anterior, L lateral

Fig. 11.21
Normal transverse neutral view. (a) The leg is now in the physiologic neutral position with the transducer positioned laterally over the hip. (b) Diagram shows the femoral head (asterisk), pubis (arrow), triradiate cartilage (open arrowhead), and ischium (white arrowhead). (c) On the US, the femoral head (star) is centered over the triradiate cartilage (open arrow) with the ischium (i) posterior and the pubis (p) anterior. Note the cartilage overlying the pubic bone is thicker (arrow) than that overlying the ischium. The triradiate cartilage allows through transmission of sound. A anterior, L lateral
Similar to the Barlow maneuver, the Harcke technique uses pistoning and adduction stress in the flexion view to detect instability. With stress, the unstable hip migrates away from the acetabulum laterally and posteriorly to a variable degree. Echogenic soft tissue then fills the space between the acetabulum and the femoral head. In subluxation, the femoral head remains in partial contact with the acetabulum (Fig. 11.22), whereas in dislocation, it is entirely outside the acetabulum (Fig. 11.23). It is important to examine the abnormal hip at rest and then with stress in order to determine the severity of displacement. If the hip is abnormal, it should be flexed and abducted to determine reducibility. Mild displacement with or without stress is normal in the first 3–4 weeks of life, but after this period, such movement is considered abnormal (although laxity may persist for a few more weeks in breech babies).
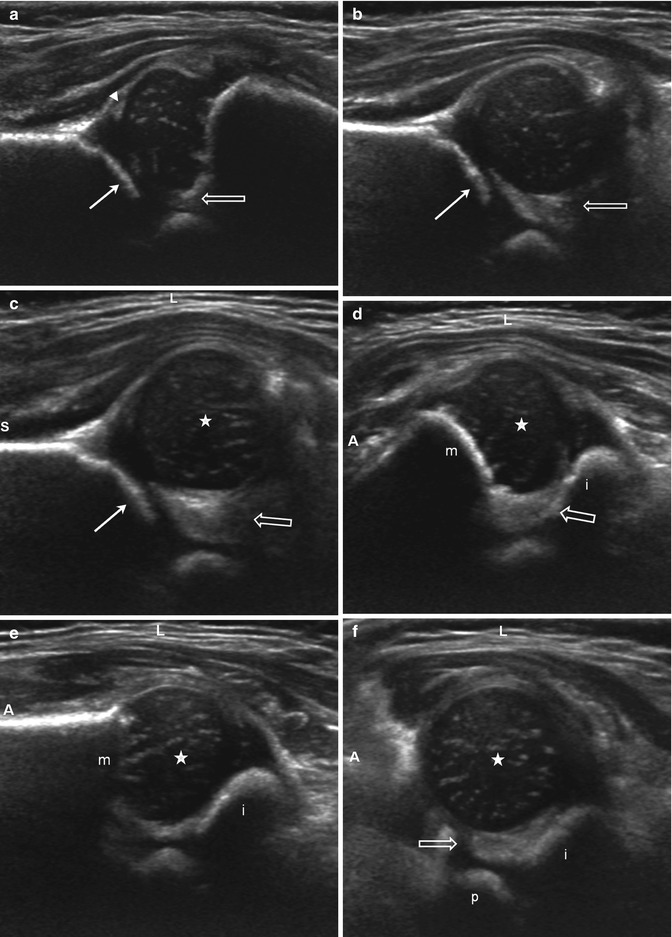
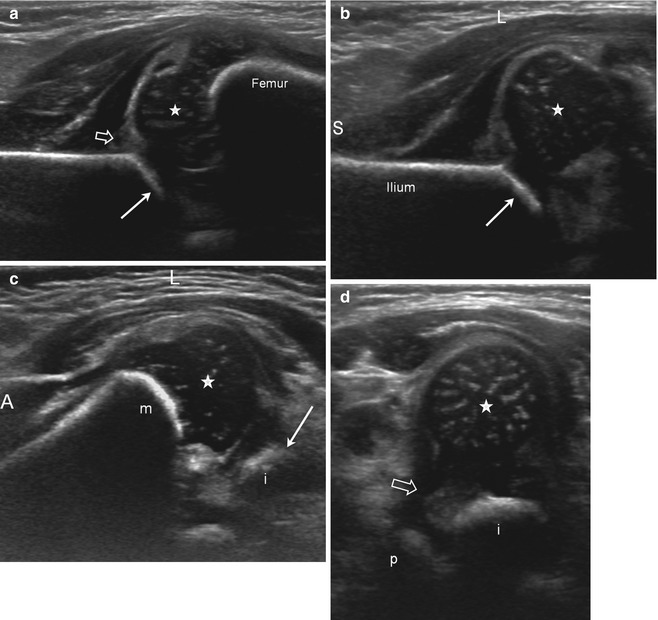

Fig. 11.22
US of subluxation (S superior, L lateral, A anterior). (a) Coronal neutral view shows mild lateral displacement of the femoral head with echogenic fibrofatty tissue (open arrow) between the femoral head and the acetabulum (arrow). The acetabular roof is angled and irregular consistent with dysplasia. Note the echogenic tip of the labrum (arrowhead). (b) Coronal flexion view, without stress. The femoral head subluxates laterally, creating space lateral to the acetabulum (arrow), which fills with echogenic fibrofatty tissue (open arrow). Again, note the irregularity of the acetabular roof. (c) Coronal flexion view with adduction stress. The gap and echogenic material (open arrow) between the femoral head (star) and the acetabulum (arrow) are further increased, but the femoral head still contacts the acetabulum. (d) Transverse flexion view in adduction. The femoral head (star) is displaced laterally with echogenic material (open arrow) between the femoral head and the medial acetabulum (open arrow). The ischium (i) is posterior and metaphysis (m) anterior. (e) Transverse flexion view in abduction. The femoral head (star) is now reduced, resting on the ischium (i) with no material intervening between the femoral head and the medial acetabulum (m metaphysis). (f) Transverse neutral view. The femoral head (star) is mildly laterally displaced with space and echogenic material (open arrow) between the head and the acetabulum, composed of the ischium (i) posteriorly and the pubis (p) anteriorly

Fig. 11.23
US of dislocation (S superior, L lateral, A anterior). (a) Coronal neutral view shows the femoral head (star) lateral to the acetabulum (arrows). The labrum (open arrow) is echogenic and thickened; the acetabulum is angled and incompletely seen. (b) Coronal flexion view. The femoral head (star) is displaced laterally and uncovered by the acetabulum (arrow). The labrum again appears abnormal, and the shallow acetabulum is angled (arrow). (c) Transverse flexion view. The femoral head (star) is displaced laterally, perched on the margin of the posterior acetabulum, or “posterior lip” (arrow) formed by the ischium (i) (m metaphysis). (d) Transverse neutral view. The femoral head (star) is laterally displaced well away from the acetabulum, composed of the ischium (i) and pubis (p). Abundant echogenic soft tissue intervenes (open arrow)
The acetabular d/D measurement, obtained in the coronal plane, evaluates acetabular coverage; the appearance is comparable to a frontal radiograph (Fig. 11.24) (Table 11.2) [43, 44]. The d/D measurement also correlates with the Graf alpha angle [45].
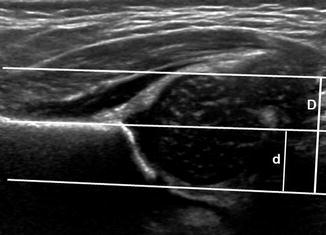

Fig. 11.24
Normal coronal flexion US showing d/D ratio. Three lines parallel to the iliac wing define the depth of the acetabulum (d, short line) and the diameter of the femoral head (D, long line). The most lateral is tangent to the lateral margin of the femoral head. The middle line runs along the edge of the straight, echogenic iliac bone. The most medial line is tangent to the medial margin of the femoral head. The d/D ratio is normally over 58 %
Table 11.2
Ultrasound measurements. Acetabular (d/D ratio)
Acetabular coverage (d/D ratio) (Fig. 11.24) | |
|---|---|
Purpose | To estimate the acetabular coverage by comparing the diameter of the femoral head to the depth of the acetabular cup |
Definition | In the coronal flexion view, with the hip normally positioned in the acetabulum, three parallel lines are drawn, one along the ilium (iliac line) and the other 2 parallel to it, one tangent to the lateral aspect of the femoral head, and one tangent to the junction of the medial femoral head and the acetabular fossa. The distance from the medial line and the iliac line is d, and the distance from the medial line and the lateral line is D. The d/D ratio is multiplied by 100 and represents the percentage of the femoral head covered by the bony acetabulum |
Note | When the hip is not well positioned in the acetabulum or the acetabulum is dysplastic, 4 parallel lines must be drawn, one along the ilium and one parallel to it in the deepest portion of the acetabulum, representing d, the depth of the acetabulum, and the other two tangential to the medial and lateral aspects of the femoral head, representing D, the width of the femoral head |
Values | |
Graf Static Hip Ultrasound
Coronal views are obtained with the patient in the decubitus position, typically in an immobilizing device. Landmarks that must be included are the straight lateral margin and inferior tip of the ilium, as well as the fibrocartilaginous tip of the labrum. The slope of the acetabulum, the alpha and beta angles, and the labral coverage are measured. The normal alpha angle is 60° or more, and the normal beta angle is less than 55° (Fig. 11.25) (Table 11.3). The Graf classification system primarily uses the alpha angle for prognosis and treatment. This method is employed extensively in Europe and in many centers in the United States as well.
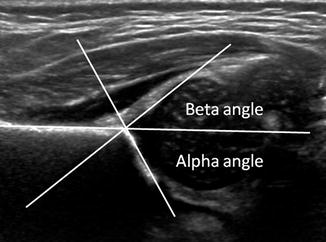

Fig. 11.25
Normal coronal neutral US shows Graf angles. The alpha angle is normally greater than 60°
Purpose | To measure the development of the acetabulum as a guide for treatment and prognosis of DDH |
Definition | A coronal view is obtained in the neutral position with the critical landmarks of the straight iliac line, the fibrocartilaginous tip of the labrum, and the inferior tip of the ilium. A line is drawn along the straight portion of the iliac bone (iliac line). A line is then drawn connecting the inferior margin of the ilium and the lateral margin of the acetabular roof to create the alpha angle. A second line can be drawn connecting the fibrocartilaginous tip of the labrum and the lateral margin of the acetabulum, forming the beta angle |
Note | All landmarks must be present on the same image to ensure that the measurements are in the correct plane |
Graf type | Values [46, 47] | |
|---|---|---|
Alpha angle | Beta angle | |
I | ≥60° (normal) | |
Ia | <55° | |
Ib | >55° | |
IIa | 50–59° | |
IIb | 50–59° | |
IIc | 43–49° | |
D (“about to decenter”) | 43–49° | <77° |
III (types IIIa and IIIb are distinguished on the grounds of structural alteration of the cartilaginous roof)
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

| ||