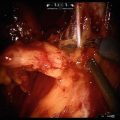
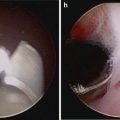
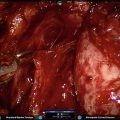
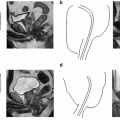
Fig. 5.1
Transperitoneal trocar placement. From Lei Y., et al. Athermal Division and Selective Suture Ligation of the Dorsal Vein Complex During Robot-Assisted Laparoscopic Radical Prostatectomy: Description of Technique and Outcomes. Eur Urol 2011;59: 235–243. Reprinted with permission from Elsevier Limited
After abdominal wall hair is clipped in the right upper quadrant and the lower abdomen, the Veress needle is inserted 1–2 cm supraumbilically in the midline. The peritoneum is then insufflated to 15 mm of mercury, the Veress needle removed, and the scalpel used to make a 12 mm vertical skin incision at the site of the Veress needle. Electrocautery is used to dissect through the dermis. We then place a 12 mm port by feel, with a “give” indicating the tip of the port is through the posterior rectus sheath. In men with prior abdominal surgery, the Hassan technique is used to place the initial 12 mm port. The zero degree robotic lens and camera are then inserted through this supraumbilical 12 mm port to guide placement of the remaining trocars. Another 12 mm port is placed 1–2 cm medial to the right anterior iliac spine for the assistant laparoscopic graspers and placement of the Hem-o-Lok® (Medline Industries, Inc. Mundelein, Illinois). Care is taken to ensure that this 12 mm port is not placed lateral to the anterior axillary line, as this will force the assistant to come in at an upward trajectory before dropping down into the surgical field. Additionally, insufflation tubing is placed on this 12 mm port. Next, a 5 mm trocar is placed in the right upper quadrant, medial to the lateral rectus margin. This serves as the point of access for the assistant surgeon’s bariatric tip suction irrigator. Care is taken to place this port at least 2 cm below the costal margin, as placement closer to the costal margin may inhibit the fulcrum of the suction irrigator into the pelvis.
Next, the 8 mm robotic trocars are placed in the same horizontal plane as the assistant 12 mm port site. These three robotic trocars are placed at the right (number 1 robotic arm) and left rectus margin (number 2 robotic arm), and 1–2 cm medial to the left anterior iliac spine (number 3 robotic arm). For men with prior appendectomy, it is often necessary to place the left lower quadrant robotic trocars initially. Laparoscopic scissors are then inserted through these ports to a perform lysis of adhesions, in order to safely place the right lower quadrant trocars.
Dropping the Bladder, Entry into the Space of Retzius
While we initially conformed to the traditional Montsouris approach of performing a posterior dissection of the peritoneal cul-de-sac to dissect out the seminal vesicles and ligate the vas deferens before dropping the bladder, we converted to the anterior approach for several reasons [8]. First, in men with extensive prior abdominal surgery, the posterior approach is incompatible with an extraperitoneal approach. Second, the posterior approach ties up robotic arm number 3 to retract the sigmoid colon as well as an assistant’s instrument to hold up the anterior leaflet of the cut peritoneal edge. Therefore exposure may be more challenging, necessitating more “one-handed” dissection and greater use of monopolar cautery. Finally, incision of the peritoneal cul-de-sac, while not time consuming, is an additional step that is not required with the anterior approach.
With the curved monopolar scissors in robotic arm number 1, the Maryland bipolar dissector in robotic arm number 2, and the ProGrasp™ in robotic arm number 3, we incise the medial umbilical ligaments and the urachus as cephalad as possible. Care must be taken to fully cauterize these structures, as patent vessels may traverse the medial umbilical ligaments. We then downwardly retract the cut edge, and dissect beneath the preperitoneal fat in the midline of the fibro-areolar plane until we encounter the symphysis pubis. Next, the peritoneal incision is extended posterolaterally from the incised medial umbilical ligament to the vas deferens at its intersection with the iliac vessels. This is repeated on the contralateral side. We deliberately incise the peritoneum medial to the internal ring to avoid weakening the abdominal wall and increasing the susceptibility to an inguinal hernia. The bladder is retracted medially, away from the iliac vessels and pelvic sidewall, and this firm traction facilitates identification of the fibro-areolar, anatomic plane lateral to the bladder. The final maneuver to mobilize the bladder away from the sidewall is accomplished by turning the ipsilateral robotic instrument tip at a right angle toward the symphysis pubis. The rounded, hinged part of the endowrist is used to sweep between the bladder and the pelvic sidewall toward the ipsilateral shoulder, stopping where the common iliac artery bifurcates. After fully mobilizing the bladder, the area over the mid-prostate is then de-fatted using bipolar cautery. We do not defat over the apex of the prostate, as this is unnecessary with our antegrade approach. If anything, this maneuver increases the risk of venous bleeding, which requires additional time to control.
Bladder Neck Sparing Dissection
In contrast to our initial description [9, 10], we no longer suture ligate the mid-prostate or anterior bladder prior to bladder neck dissection. The ProGrasp™ (in robotic arm number 3 arm) grasps and tents the bladder wall anteriorly and cephald to identify the detrusor apron’s intersection with the vesico-prostatic junction (Fig. 5.2). Staying midline, sharp dissection is used through the connective tissue of the detrusor apron until bladder fibers are seen. We prefer sharp dissection, since monopolar cautery may obliterate or obscure these fibers. Bipolar electrocautery is used in short bursts for hemostasis. After reaching the bladder fibers, the prostatic contour in the sagittal plane is followed proximally to the bladder neck. This incision is extended lateral in an arced fashion to avoid vessels that traverse the prostate from the lateral pedicle to the dorsal vascular complex.
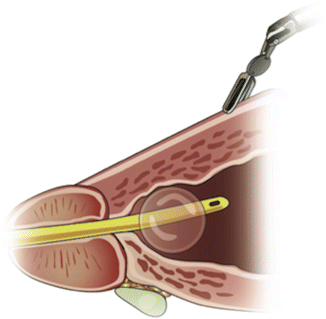

Fig. 5.2
Robotic arm number 3 provides upward tension on the prostate base while the assistant grasps the posterior lip of the bladder neck to provide countertension. From Freire MP., et al. Anatomic Bladder Neck Preservation During Robotic-assisted Laparoscopic Radical Prostatectomy: Description of Technique and Outcomes. Eur Urol. 2009;56(6):972–80. Reprinted with permission from Elsevier Limited
Next, blunt dissection is performed in a caudal direction over the anterior bladder neck, identifying the vertical fibers of the prostatic urethra. We then perform blunt dissection lateral to the bladder neck by opening the Maryland dissector and manipulating the scissors caudally, resulting in a triangular spread on the lateral lobes of the prostate. This also defines the funneled shape of the bladder neck and its junction with the prostatic urethra. The bladder neck is opened anteriorly, and the urethral catheter is withdrawn after deflating the balloon. The posterior bladder mucosa is then incised using monopolar electrocautery, while the ProGrasp™ is used to elevate the prostatic base, avoiding the need for catheter manipulation during this step. The surgical assistant then provides countertraction on the bladder neck to allow the dissection to continue posteriorly to the detrusor apron. Dissecting laterally before identification of the detrusor apron may result in inadvertent cystotomy or ureteral injury.
After identification of the posterior longitudinal detrusor layer, we turn the dissection laterally until adipose tissue is encountered. The adipose tissue is lateral to the bladder neck and found at the cephalad portion of the endopelvic fascia. This landmark was originally named the fat pad of Whitmore, and it defines the posterolateral bladder neck dissection boundary, as the neurovascular bundle is in very close proximity to the lateral prostatic pedicle [9, 10]. Complete division of the bladder neck to the fat pad of Whitmore allows for better exposure during the seminal vesicle dissection. The posterior detrusor layer is then opened as posteriorly as possible, close to the seminal vesicle tips, and the vasa deferentia are identified behind a layer of adipose tissue. This layer is thicker in obese men and almost nonexistent in very thin individuals.
Seminal Vesicle and Posterior Dissection
After identification of the vasa, the ProGrasp™ lifts them anteriorly, while the Maryland bipolar dissects around them to create space for a purple Hem-o-lok clip. Care must be taken to avoid closing the clip on the seminal vesicle located behind the vasa. After clipping each vas but prior to transection, assistant grasper countertraction is provided below the clip and the vas pulled toward the assistant trocar. This exposes the tip of the seminal vesicle, allowing for safe transection of the vas to proceed. The artery of the vas usually courses between the vas and medial aspect of the seminal vesicle. Hemostasis is controlled by either the aforementioned clip or with bipolar cautery prior to division. After dividing each vas, the ProGrasp™ grasps the proximal aspect of the seminal vesicle and lifts it anteriorly. Blunt dissection is used to define the medial and typically avascular border of the seminal vesicle [11]. The arterial blood supply of the seminal vesicle originates inferolaterally, and bipolar cautery is used sparingly away from the seminal vesicle tips to control arterioles (Fig. 5.3).
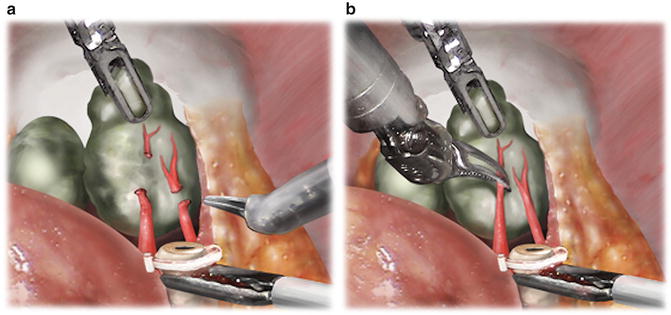

Fig. 5.3
(a) Fourth-arm ProGrasp™ anterior traction and assistant laparoscopic grasper countertraction facilitate the high isolation of arterioles proximal to the seminal vesicle tip. From Kowalczyk KJ., et al. Stepwise Approach for Nerve Sparing Without Countertraction During Robot-assisted Radical Prostatectomy: Technique and Outcomes. Eur Urol. 2011;60(3):536–47. Reprinted with permission from Elsevier Limited. (b) Bipolar 25-W current fulguration of arterioles prior to cut-and-peel technique to divide and bluntly sweep arterioles beyond the seminal vesicle tip. From Kowalczyk KJ., et al. Stepwise Approach for Nerve Sparing Without Countertraction During Robot-assisted Radical Prostatectomy: Technique and Outcomes. Eur Urol. 2011;60(3):536–47. Reprinted with permission from Elsevier Limited
Particularly for larger seminal vesicles, the ProGrasp™ must be continuously repositioned as progress is made toward the seminal vesicle tip. The temptation to dissect the lateral proximal border of the seminal vesicle to its origin must be avoided, as this may result in capsular incision and/or bleeding from the lateral pedicle vessels. The dissection is ultimately finished when the lateral pedicles are ligated and divided in a subsequent step [11].
Attention is then turned posteriorly. The ProGrasp™ again grasps the seminal vesicles and lifts them anteriorly. Denonvilliers’ fascia is incised in the midline and blunt dissection is used to define the posterior prostate contour (Fig. 5.4). The nerve-sparing dissection plane is recognized by a layer of glistening Denonvilliers’ fascia coursing above the pre-rectal fat [12]. This plane of dissection is continued distally toward the apex of the prostate and then laterally until the medial border of the neurovascular bundle is encountered. The neurovascular bundle may be landmarked by veins coursing longitudinally along the prostate. Alternatively, for men with high volume, high Gleason grade disease for whom interfascial or extrafascial nerve sparing is preferable, this dissection plane is carried out beneath Denonvilliers’ fascia onto the pre-rectal fat. Greater prostatic volume impedes visualization and limits the extent of the posterior dissection distally. This requires subsequent rotation of the prostate after antegrade neurovascular bundle release to complete the apical dissection posteriorly [13].
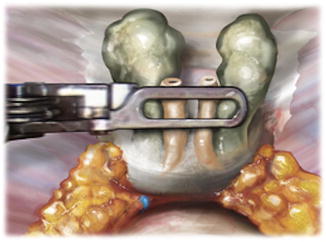

Fig. 5.4
After seminal vesicle dissection and prior to defining the anterior prostatic contour (endopelvic fascia intact), the posterior dissection is performed. Denonvilliers’ fascia is separated posteriorly from the prostatic fascia in the midline, and the posterior contour is defined. This dissection is carried out lateral to the lateral pedicle fat pad proximally. Distally, veins that provide the landmark of the medial border of the neurovascular bundle are commonly encountered at the mid- and apical prostate and serve as the lateral border of the dissection. From Lei Y., et al. Athermal Division and Selective Suture Ligation of the Dorsal Vein Complex During Robot-Assisted Laparoscopic Radical Prostatectomy: Description of Technique and Outcomes. Eur Urol 2011;59: 235–243. Reprinted with permission from Elsevier Limited
Lateral Pelvic Fascia Separation and Development of the Anterior Prostatic Contour
At our institution, we prefer to perform right versus left nerve sparing first because of the better working angles with the robotic scissors already entering to the right of midline. The left-sided nerve sparing is technically easier after increased prostate mobility from releasing the right-sided attachments. Beginning at the right mid-prostate, a “prostatic rub” is used medial to the fascial tendinous arch to split the pelvic fascia, leaving the levator fascia laterally and the prostatic fascia with the prostate (Fig. 5.5) [12]. “Rubbing” proximally toward the base helps define the distal fold of the lateral pedicle. The thickness of the prostatic fascia is not uniform and in men with more translucent prostatic fascia, fibro-adipose tissue is seen underlying this fascia. The junction between the medial aspect of the fibro-adipose tissue and prostate capsule defines the intrafascial dissection plane. Incision of the outer prostatic fascia allows the medial edge of the neurovascular bundle to be identified. We do not routinely perform this move, however, especially when prominent capsular veins course below the outer prostatic fascia. While pneumoperitoneum typically results in thrombosis and hemostasis if inadvertent venotomies occur, avoiding these veins is prudent to prevent venous oozing that impedes visualization and takes time to control [11]. After completing the lateral pelvic fascia separation on the right, we then proceed in a similar manner on the left.
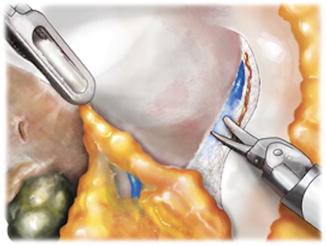

Fig. 5.5




Prostatic rub used for periprostatic fascia separation. Nerve bundle components are encountered on the medial aspect of the levator fascia and pushed laterally. The outer prostatic fascia remains medically on the prostate distal to the lateral pedicle and the fat pad located posterolaterally between the bladder and the prostate. In thinner men with finer or more translucent prostatic fascia, fibroadipose tissue may be seen underlying the outer prostatic fascia. From Lei Y., et al. Athermal Division and Selective Suture Ligation of the Dorsal Vein Complex During Robot-Assisted Laparoscopic Radical Prostatectomy: Description of Technique and Outcomes. Eur Urol 2011;59: 235–243. Reprinted with permission from Elsevier Limited
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree