Congenital |
Gray matter heterotopia |
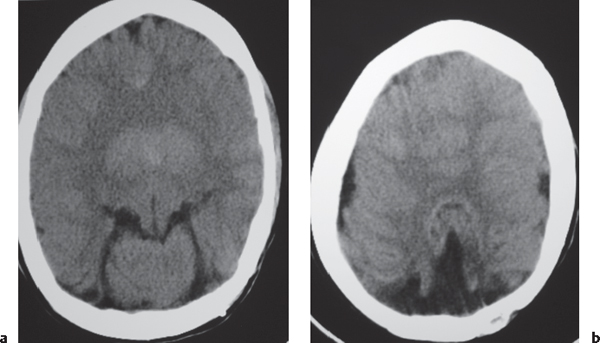
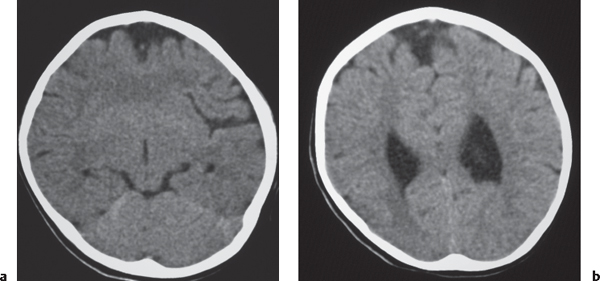
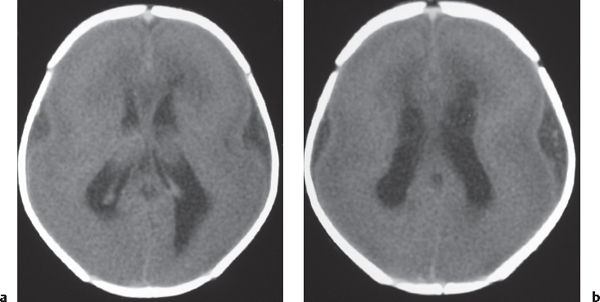
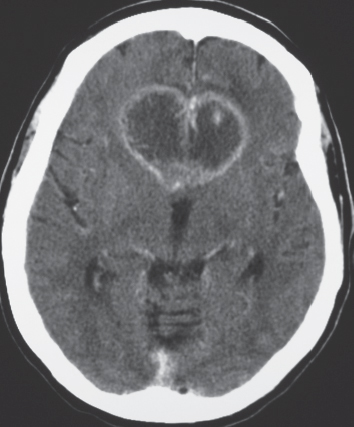
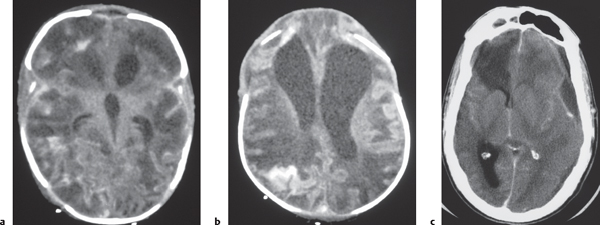
Laminar heterotopia appears as a band or bands of gray matter attenuation within the cerebral white matter (see Fig. 1.7 , p. 8).
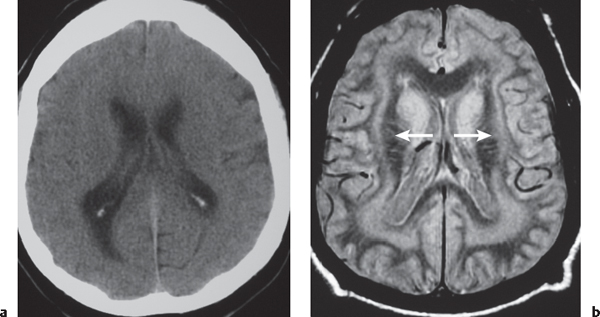
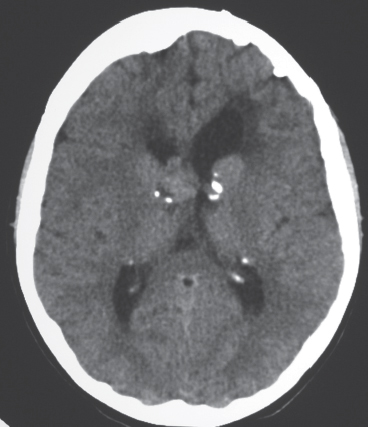
Nodular heterotopia appears as one or more nodules of gray matter attenuation along the ventricles (see Fig. 1.8 , p. 8 ) or within the cerebral white matter (see Fig. 1.9 , p. 9 ).
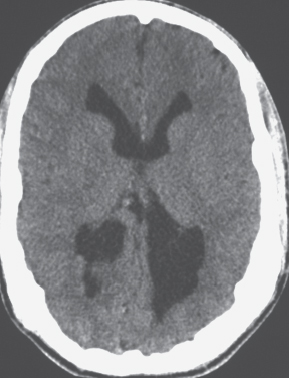
Focal subcortical heterotopia can be seen as irregular nodular or multinodular masslike zones with gray matter attenuation in subcortical regions (see Fig. 1.9 , p. 9 ). |
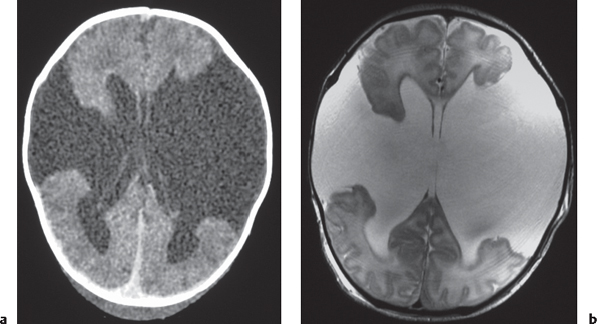
Disorder of neuronal migration (weeks 7–22 of gestation) in which a collection or layer of neurons is located between the ventricles and cerebral cortex. Can have a bandlike (laminar) or nodular appearance isodense to gray matter; may be unilateral or bilateral. Associated with seizures and schizencephaly. |
Unilateral hemimegalencephaly
Fig. 1.11 , p. 9 |
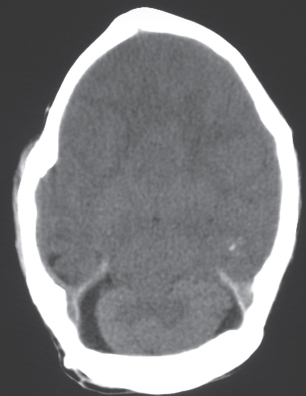
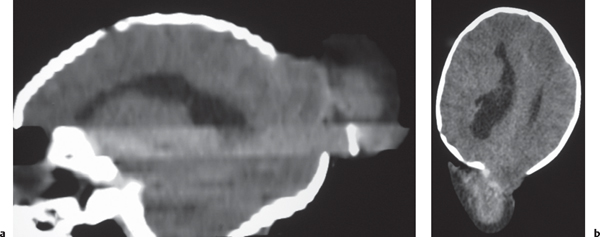
Nodular or multinodular region of gray matter heterotopia involving all or part of a cerebral hemisphere with associated enlargement of the ipsilateral lateral ventricle and hemisphere. |
Neuronal migration disorder associated with hamartomatous overgrowth of a portion of or the whole hemisphere. |
Neoplastic astrocytoma |
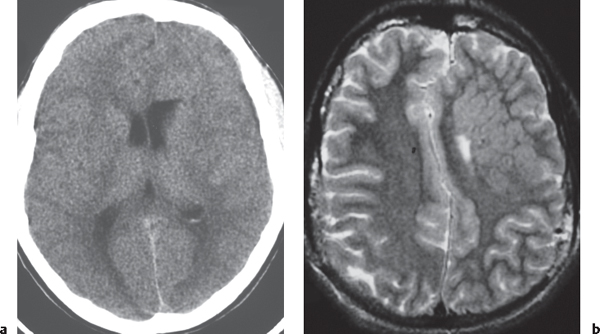
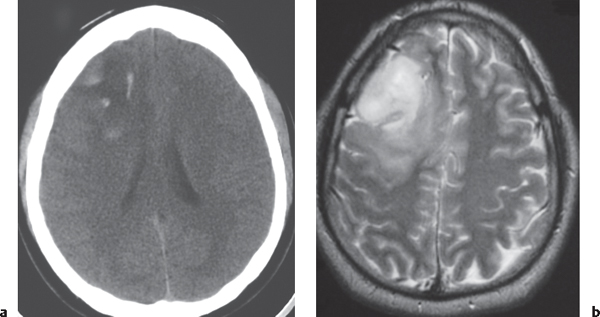
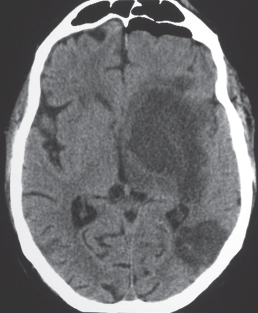
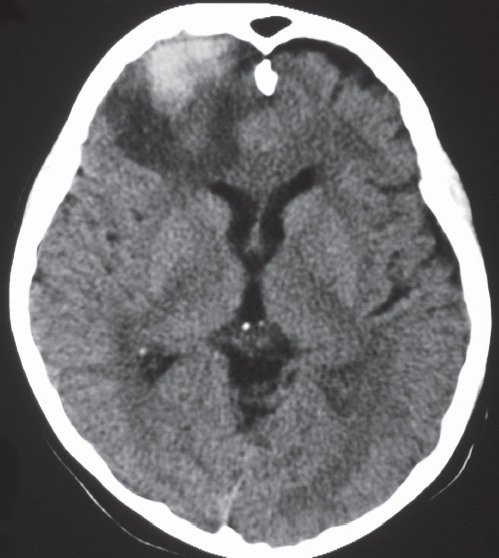
Low-grade astrocytoma: Focal or diffuse mass lesion usually located in white matter with low to intermediate attenuation, with or without mild contrast enhancement. Minimal associated mass effect.
Fig. 1.23
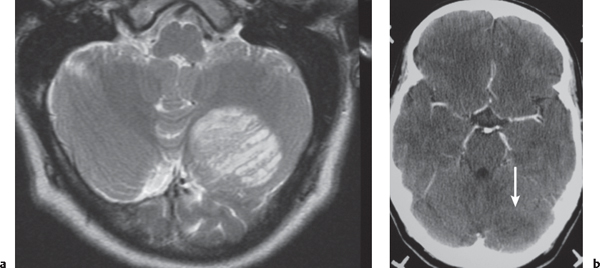
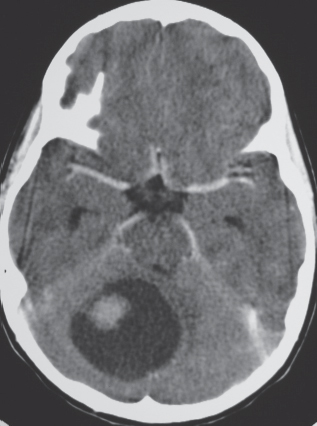
Juvenile pilocytic astrocytoma subtype: Solid/cystic focal lesion with low to intermediate attenuation, usually with prominent contrast enhancement. Lesions located in the cerebellum, hypothalamus, adjacent to third or fourth ventricles, and brainstem.
Fig. 1.24
Gliomatosis cerebri: Infiltrative lesion with poorly defined margins with mass effect located in the white matter, with low to intermediate attenuation. Usually no contrast enhancement until late in disease. Anaplastic astrocytoma: Often irregularly marginated lesion located in white matter with low to intermediate attenuation, with or without contrast enhancement.
Fig. 1.25a, b |
Low-grade astrocytoma: Often occurs in children and adults (20–40 y). Tumors comprised of well-differentiated astrocytes. Association with neurofibromatosis type 1, 10-y survival; may become malignant. Juvenile pilocytic astrocytoma subtype: Common in children; usually favorable prognosis if totally resected. |
|
Gliomatosis cerebri: Diffusely infiltrating astrocytoma with relative preservation of underlying brain architecture. Imaging appearance may be more prognostic than histologic grade; ~2-y survival. |
Anaplastic astrocytoma: Intermediate between low-grade astrocytoma and glioblastoma multiforme; ~2-y survival. |
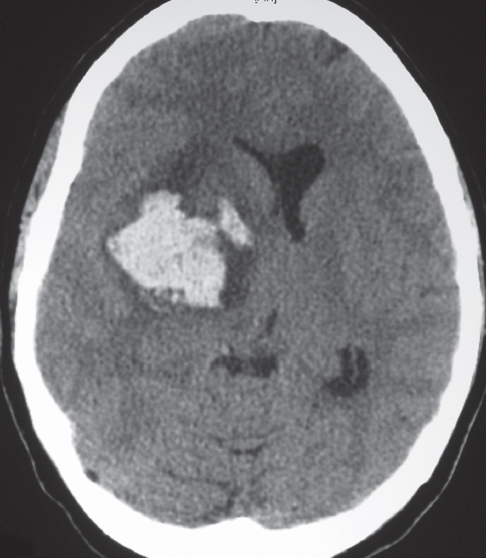
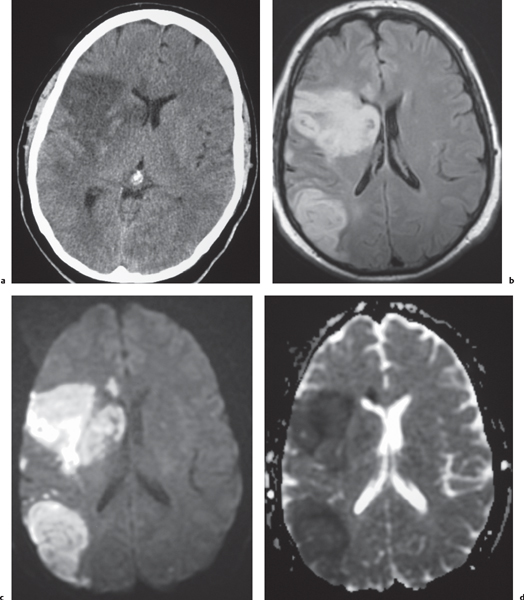
Glioblastoma multiforme
Fig. 1.26 |
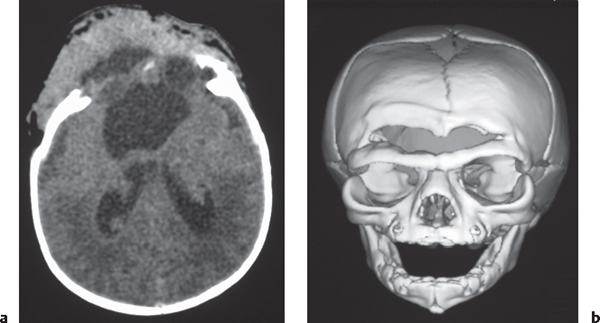
Irregularly marginated mass lesion with necrosis or cyst, mixed low and intermediate attenuation, with or without hemorrhage, prominent heterogeneous contrast enhancement, peripheral edema; can cross the corpus callosum. |
Most common primary central nervous system (CNS) tumor in adults, highly malignant neoplasms with necrosis and vascular proliferation, usually in patients older than 50 y; extent of lesion underestimated by CT; survival < 1 y. |
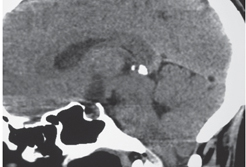
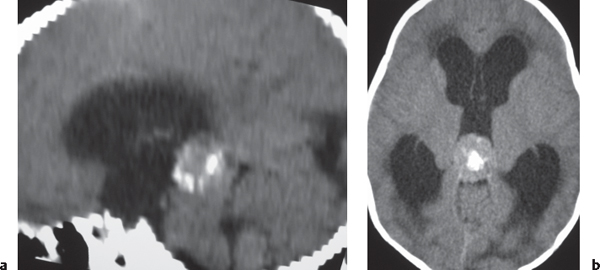
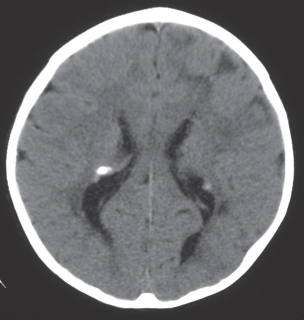
Giant cell astrocytoma/tuberous sclerosis
Fig. 1.27 |
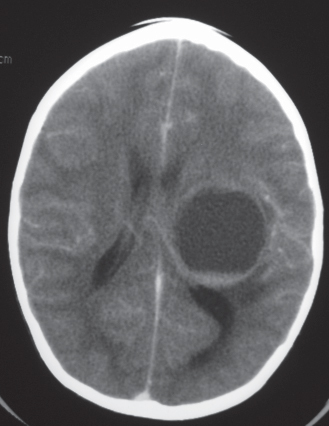
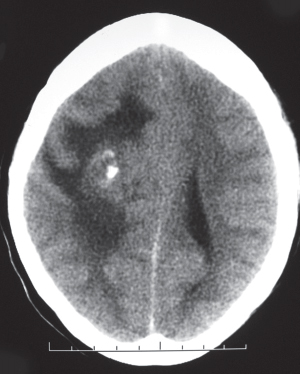
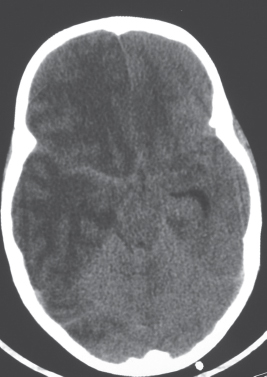
Circumscribed lesion located near the foramen of Monro with mixed low to intermediate attenuation, with or without cysts and/or calcifications, and heterogeneous contrast enhancement. |
Subependymal hamartoma near the foramen of Monro; occurs in 15% of patients younger than 20 y with tuberous sclerosis. Slow-growing lesions can progressively cause obstruction of CSF flow through the foramen of Monro; long-term survival usual if resected. |
Pleomorphic xanthoastrocytoma |
Circumscribed supratentorial lesion involving the cerebral cortex and white matter, low to intermediate attenuation, with or without cyst (s), heterogeneous contrast enhancement, with or without enhancing mural nodule associated with cyst. |
Rare type of astrocytoma occurring in young adults and children; associated with seizure history. |
Oligodendroglioma
Fig. 1.28a, b |
Circumscribed lesion with mixed low to intermediate attenuation, sites of clumplike calcification, and heterogeneous contrast enhancement; involves white matter and cerebral cortex; can cause chronic erosion of the inner table of the calvarium. |
Uncommon slow-growing gliomas with usually mixed histologic patterns (astrocytoma, etc.). Usually in adults older than 35 y; 85% supratentorial. If low grade, 75% 5-y survival; higher grade lesions have a worse prognosis. |
Central neurocytoma |
Circumscribed lesion located at the margin of the lateral ventricle or septum pellucidum with intraventricular protrusion, heterogeneous low and intermediate attenuation, with or without calcifications and/or small cysts, heterogeneous contrast enhancement. |
Rare tumors that have neuronal differentiation; imaging appearance similar to intraventricular oligodendrogliomas. Occur in young adults. Benign slow-growing lesions. |
Ganglioglioma, ganglioneuroma, gangliocytoma |
Circumscribed tumor, usually supratentorial, often temporal or frontal lobes, low to intermediate attenuation, with or without cysts, with or without contrast enhancement. |
Ganglioglioma (contains glial and neuronal elements), ganglioneuroma (contains only ganglion cells). Uncommon tumors; seen in young adults younger than 30 y. Seizure presentation, slow-growing neoplasms. Gangliocytoma (contains only neuronal elements and dysplastic brain tissue). Favorable prognosis if completely resected. |
Ependymoma
Fig. 1.29 |
Circumscribed lobulated supratentorial lesion, often extraventricular, with or without cysts and/or calcifications, low to intermediate attenuation, variable contrast enhancement. |
Occurs more commonly in children than adults, one third supratentorial, two-thirds infratentorial; 45% 5-y survival. |
Pineal gland tumors
Fig. 1.30a, b |
Tumors often have intermediate attenuation to intermediate to slightly high attenuation, with contrast enhancement, with or without central and/or peripheral calcifications. Malignant tumors are often larger than benign pineal lesions (pineocytoma), as well as heterogeneous attenuation and contrast enhancement pattern, with or without leptomeningeal tumor. |
Pineal gland tumors account for 8% of intracranial tumors in children and 1% of tumors in adults; 40% of tumors are germinomas, followed by pineoblastomas and pineocytomas, teratomas, choriocarcinomas, endodermal sinus tumors, astrocytomas, and metastatic tumors. |
Hamartoma/tuberous sclerosis
Fig. 1.31 |
Cortical-subcortical lesion with variable attenuation, calcifications in 50% of older children; contrast enhancement uncommon.
Subependymal hamartomas: Small nodules located along and projecting into the lateral ventricles; calcification and contrast enhancement common. |
Cortical and subependymal hamartomas are nonmalignant lesions associated with tuberous sclerosis. Tuberous sclerosis is an autosomal dominant disorder associated with hamartomas in multiple organs. |
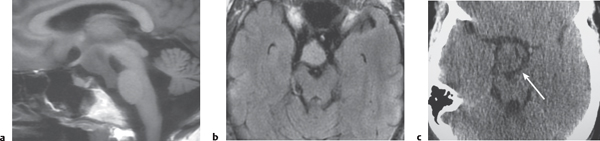
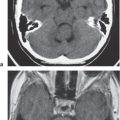
Hypothalamic hamartoma
Fig. 1.32a–c |
Sessile or pedunculated lesions at the tuber cinereum of the hypothalamus; often intermediate attenuation similar to gray matter; typically no contrast enhancement; rarely contain cystic and/or fatty portions. |
Usually occur in children with isosexual precocious puberty (0–8 y) or seizures (gelastic or partial complex) in second decade; congenital/developmental heterotopia/hamartoma (nonneoplastic lesions). |
Lipoma
Fig. 1.33 |
CT: Lipomas have low attenuation equal to fat elsewhere in the field of view.
MRI: Lipomas have signal isointense to subcutaneous fat on T1-weighted images (high signal) and on T2-weighted signals; signal suppression occurs with frequency-selective fat saturation techniques or with a short time to inversion recovery (STIR) method; typically no gadolinium contrast enhancement or peripheral edema. Lipomas can be nodular or curvilinear. Lipomas can occur in many locations, commonly the corpus callosum, cerebellopontine angle cistern, and tectal plate. |
Benign fatty lesions resulting from congenital malformation often located in or near the midline; may contain calcifications and/or traversing blood vessels. |
Primitive neuroectodermal tumor
Fig. 1.34a, b |
Circumscribed or invasive lesions, low to intermediate attenuation; variable contrast enhancement, frequent dissemination into the leptomeninges. |
Highly malignant tumors located in the cerebrum, pineal gland, and cerebellum that frequently disseminate along CSF pathways. |
Dysembryoplastic neuroepithelial tumor
Fig. 1.35a, b |
Circumscribed lesions involving the cerebral cortex and subcortical white matter, low to intermediate attenuation, with or without small cysts; usually no contrast enhancement. |
Benign superficial lesions commonly located in the temporal or frontal lobes. |
Lymphoma
Fig. 1.36 |
Primary CNS lymphoma: Focal or infiltrating lesion located in the basal ganglia, periventricular regions, or posterior fossa/brainstem; low to intermediate attenuation; with or without hemorrhage/necrosis in immuno-compromised patients; usually show contrast enhancement. Diffuse leptomeningeal contrast enhancement is another pattern of intracranial lymphoma. |
Primary CNS lymphoma more common than secondary, usually in adults older than 40 y. B-cell lymphoma is more common than T-cell lymphoma. Increasing incidence related to the number of immunocompromised patients in the population. CT imaging features of primary and secondary lymphoma of brain overlap. Intracranial lymphoma can involve the leptomeninges in secondary lymphoma > primary lymphoma. |
Hemangioblastoma |
Circumscribed tumors usually located in the cerebellum and/or brainstem; small contrast-enhancing nodule with or without cyst or larger lesion with prominent heterogeneous enhancement with or without contrast-enhancing vessels within the lesion or at the periphery, Occasionally lesions have evidence of recent or remote hemorrhage. |
Rarely occurs in cerebral hemispheres; occurs in adolescents and young and middle-aged adults. Lesions are typically multiple in patients with von HippelLindau disease. |
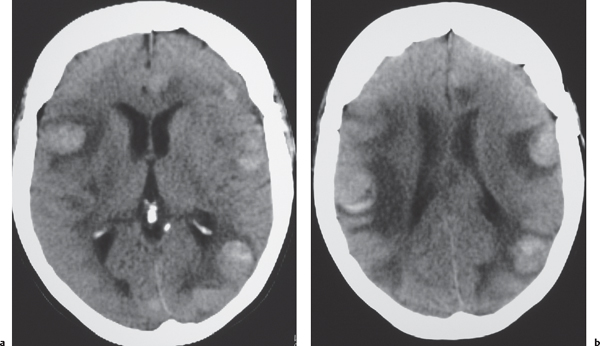
Metastases
Fig. 1.37a, b |
Circumscribed spheroid lesions in the brain; can have various intra-axial locations, often at gray-white matter junctions; usually low to intermediate attenuation; with or without hemorrhage, calcifications, or cysts; variable contrast enhancement. Often associated with adjacent low attenuation from axonal edema. |
Represent ~33% of intracranial tumors, usually from extracranial primary neoplasm in adults older than 40 y. Primary tumor source: lung > breast > gastrointestinal (GI) > genitourinary (GU) > melanoma. |
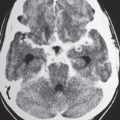
Neurocutaneous melanosis |
Extra- or intra-axial lesions usually < 3 cm in diameter with irregular margins in the leptomeninges or brain parenchyma/brainstem (anterior temporal lobes, cerebellum, thalami, and inferior frontal lobes).
CT: May show subtle hyperdensity secondary to increased melanin, with or without vermian hypoplasia, with or without arachnoid cysts, with or without Dandy-Walker malformation.
MRI: Zones with intermediate to slightly high signal on T1-weighted images secondary to increased melanin, with gadolinium contrast enhancement; with or without vermian hypoplasia, with or without arachnoid cysts, with or without Dandy-Walker malformation. |
Neuroectodermal dysplasia with proliferation of melanocytes in leptomeninges associated with large and/or numerous cutaneous nevi. May change into CNS melanoma. |
Inflammatory |
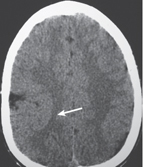
Cerebritis
Fig. 1.38a–c |
Poorly defined zone or focal area of decreased attenuation, minimal or no contrast enhancement; involves cerebral cortex and white matter for bacterial and fungal infections. |
Focal infection/inflammation of brain tissue from bacteria or fungi, secondary to sinusitis, meningitis, surgery, hematogenous source (cardiac and other vascular shunts), and/or immunocompromised status. Can progress to abscess formation. |
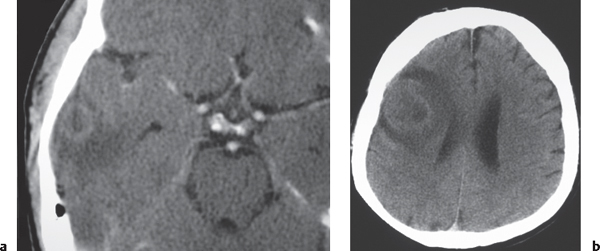
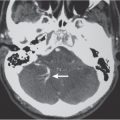
Pyogenic brain abscess
Fig. 1.39a, b |
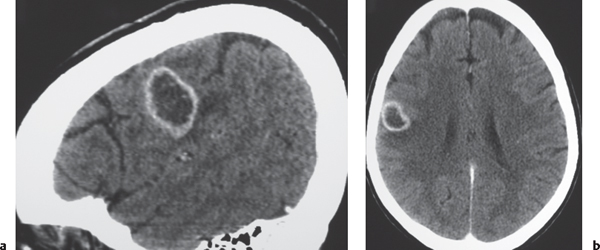
Circumscribed lesion with a central zone of low attenuation (with or without air-fluid level) surrounded by a thin rim of intermediate attenuation; peripheral poorly defined zone of decreased attenuation representing edema; ringlike contrast enhancement that is sometimes thicker laterally than medially. |
Formation of brain abscess occurs 2 weeks after cerebritis with liquefaction and necrosis centrally surrounded by a capsule and peripheral edema. Can be multiple. Complication from meningitis and/or sinusitis, septicemia, trauma, surgery, or cardiac shunt. |
Fungal brain abscess
Fig. 1.40 |
Findings can vary depending on organism; lesions occur in meninges and brain parenchyma; solid or cystic-appearing lesions with decreased attenuation, nodular or ring pattern of contrast enhancement, peripheral zone with decreased attenuation in brain lesions (edema). |
Occur in immunocompromised or diabetic patients with resultant granulomas in meninges and brain parenchyma. Cryptococcus involves the basal meninges and extends along perivascular spaces into the basal ganglia; Aspergillus and Mucor spread via direct extension through the paranasal sinuses or hematogenously and invade blood vessels, resulting in hemorrhagic lesions and/or cerebral infarcts. Coccidioidomycosis usually involves the basal meninges. |
Encephalitis
Fig. 1.41 |
Poorly defined zone or zones of decreased attenuation, minimal or no contrast enhancement; involves the cerebral cortex and/or white matter; minimal localized mass effect. Herpes simplex typically involves the temporal lobes/limbic system with or without hemorrhage; cytomegalovirus (CMV) usually in periventricular/subependymal locations.
HIV often involves periatrial white matter. |
Infection/inflammation of brain tissue from viruses, often seen in immunocompromised patients (e.g., herpes simplex, CMV, HIV, and progressive multifocal leukoencephalopathy) or immunocompetent patients (e.g., St. Louis encephalitis, eastern or western equine encephalitis, and Epstein-Barr virus). |
Tuberculoma
Fig. 1.42 |
Intra-axial lesions in cerebral hemispheres, basal ganglia, and brainstem (adults) and cerebellum (children). Lesions can have decreased attenuation, central zone of low attenuation with a thin peripheral rim of intermediate attenuation; with solid or rim pattern of contrast enhancement; with or without calcification. Meningeal lesions: Nodular or cystic zones of basilar meningeal enhancement. |
Occurs in immunocompromised patients and inhabitants of developing countries. Caseating intracranial granulomas via hematogenous dissemination; meninges > brain lesions. |
Parasitic brain lesions |
Toxoplasmosis
Fig. 1.43 |
Single or multiple solid and/or cystic-appearing lesions located in basal ganglia and/or corticomedullary junctions in cerebral hemispheres, low to intermediate attenuation; nodular or rim pattern of contrast enhancement; with or without mild peripheral low attenuation (edema). Chronic phase: calcified granulomas. |
Most common opportunistic CNS infection in patients with AIDS; caused by ingestion of food contaminated with parasites (Toxoplasma gondii). Can be seen as a congenital or neonatal infection (TORCH syndrome: toxoplasmosis, other agents, rubella, cytomegalovirus, herpes simplex). |
Cysticercosis
Fig. 1.44a, b |
Single or multiple cystic-appearing lesions in brain or meninges; acute/subacute phase: Low to intermediate attenuation, rim with or without nodular pattern of contrast enhancement, with or without peripheral low attenuation (edema). Chronic phase: calcified granulomas. |
Caused by ingestion of ova (Taenia solium) in contaminated food (undercooked pork); involves meninges > brain parenchyma > ventricles. |
Hydatid cyst |
Echinococcus granulosus: Single or rarely multiple cystic-appearing lesions with low attenuation surrounded by a thin wall; typically no contrast enhancement or peripheral edema unless superinfected; often located in vascular territory of the middle cerebral artery. Echinococcus multilocularis: Cystic (with or without multilocular) and/or solid lesions, central zone of intermediate attenuation surrounded by a slightly thickened rim, with contrast enhancement; peripheral zone of decreased attenuation (edema) and calcifications are common. |
Caused by parasites E. granulosus (South America, Middle East, Australia, and New Zealand) and E. multilocularis (North America, Europe, Turkey, and China). CNS involvement in 2% of cases of hydatid infestation. |
Inflammatory disorders |
Radiation necrosis |
Focal lesion with or without mass effect or poorly defined zone of low to intermediate attenuation, with or without contrast enhancement involving tissue (gray matter and/or white matter) in field of treatment. |
Usually occurs from 4 to 6 months to 10 y after radiation treatment; may be difficult to distinguish from neoplasm. Positron emission tomography (PET) and magnetic resonance spectroscopy might be helpful for evaluation. |
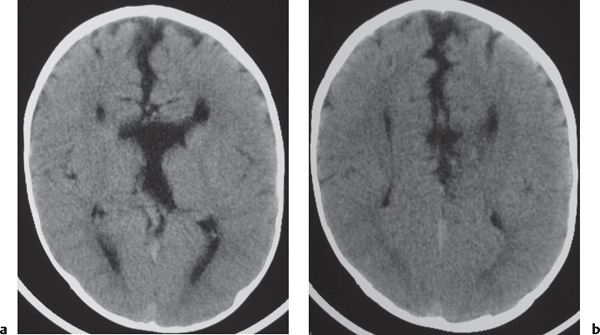
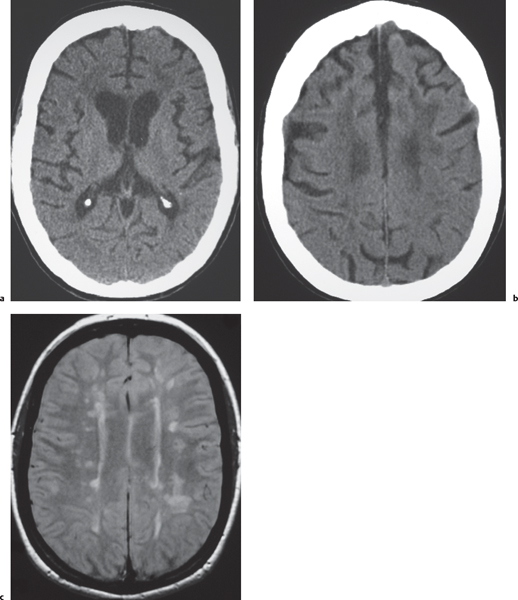
Demyelinating disease: multiple sclerosis, acute disseminated encephalomyelitis (ADEM)
Fig. 1.45a–c |
Lesions located in cerebral or cerebellar white matter, brainstem, or basal ganglia; lesions usually have low to intermediate attenuation on CT. Zones of active demyelination may show contrast enhancement and mild localized swelling.
MRI: Zones of low to intermediate signal on T1-weighted images and high signal on fluid attenuation inversion recovery (FLAIR) and T2-weighted images; with or without gadolinium contrast enhancement. Contrast enhancement can be ringlike or nodular, usually in acute/early subacute phase of demyelination. Lesions rarely can have associated mass effect simulating neoplasms. |
Multiple sclerosis is the most common acquired demyelinating disease usually affecting women (peak age 20–40 y). Other demyelinating diseases include acute disseminated encephalomyelitis/immune mediated demyelination after viral infection; toxins (exogenous from environmental exposure or ingestion of alcohol, solvents, etc., or endogenous from metabolic disorders, e.g., leukodystrophies and mitochondrial encephalopathies), radiation injury, trauma, and vascular disease. |
Sarcoidosis |
Poorly marginated intra-axial zone with low to intermediate attenuation; usually shows contrast enhancement with localized mass effect and peripheral edema. Often associated with contrast enhancement in the leptomeninges. |
Multisystem noncaseating granulomatous disease of uncertain cause that can involve the CNS in 5% to 15% of cases. Associated with severe neurologic deficits if untreated. |
Hemorrhage |
Intracerebral hemorrhage/hematoma |
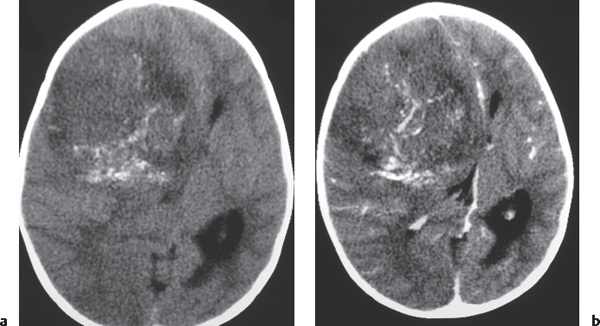
Attenuation of the hematoma depends on its age, size, location, hematocrit, hemoglobin oxidation state, clot retraction, and extent of edema.
Hyperacute phase (4–6 h): Hemoglobin primarily as diamagnetic oxyhemoglobin (iron Fe2+ state). CT: High attenuation on CT.
MRI: Intermediate signal on T1-weighted images and slightly high signal on T2-weighted images.
Acute phase (12–48 h): Hemoglobin primarily as para-magnetic deoxyhemoglobin (iron, Fe2+ state). CT: High attenuation in acute clot directly related to hematocrit, hemoglobin concentration, and high protein concentration. Hematocrit in acute clot approaches 90%.
Fig. 1.46
Fig. 1.47
Fig. 1.48
Fig. 1.49 , p. 27
MRI: Intermediate signal on T1-weighted images, low signal on T2-weighted images, surrounded by a peripheral zone of high T2 signal (edema).
Early subacute phase (> 2 d): Hemoglobin becomes oxidized to the iron Fe3+ state, methemoglobin, which is strongly paramagnetic. CT: High attenuation.
MRI: When methemoglobin is initially intracellular, the hematoma has high signal on T1-weighted images progressing from peripheral to central and low signal on T2-weighted images, surrounded by a zone of high T2 signal (edema). When methemoglobin eventually becomes primarily extra-cellular, the hematoma has high signal on T1-weighted images and high signal on T2-weighted images.
Late subacute phase (> 7 d to 6 wk): Intracerebral hematomas decrease 1.5 HU per day. Hematomas become isodense to hypodense; peripheral contrast enhancement from blood–brain barrier breakdown and vascularized capsule.
Chronic phase: Hemoglobin as extracellular methemoglobin is progressively degraded to hemosiderin.
CT: Chronic hematomas have low attenuation with localized encephalomalacia. Zones with high attenuation represent new sites of rebleeding.
MRI: Hematoma progresses from a lesion with high signal on T1- and T2-weighted images, with a peripheral rim of low signal on T2-weighted images (hemosiderin), to predominant hemosiderin composition and low signal on T2-weighted images. |
Can result from trauma, ruptured aneurysms or vascular malformations, coagulopathy, hypertension, adverse drug reaction, amyloid angiopathy, hemorrhagic transformation of cerebral infarction, metastases, abscesses, and viral infections (herpes simplex, CMV). |
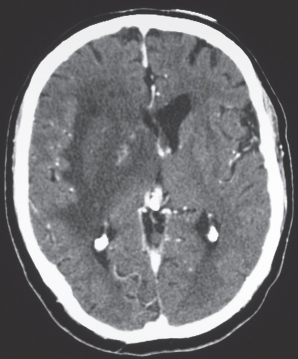
Cerebral contusions
Fig. 1.47 |
CT: Appearance of contusions is initially one of focal hemorrhage involving the cerebral cortex and subcortical white matter. Contusions eventually appear as focal superficial encephalomalacia zones. |
Contusions are superficial brain injuries involving the cerebral cortex and subcortical white matter that result from skull fracture and/or acceleration/deceleration trauma to the inner table of the skull. Often involve the anterior portions of the temporal and frontal lobes and inferior portions of the frontal lobes. |
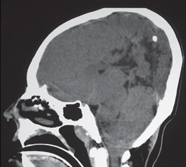
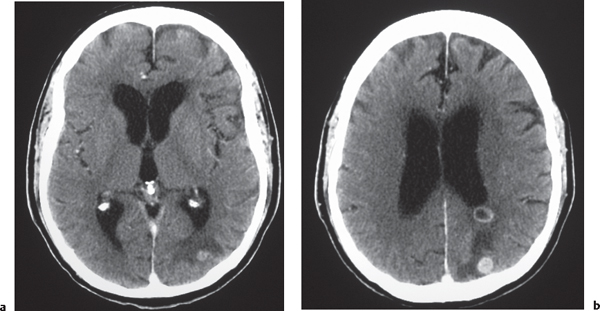
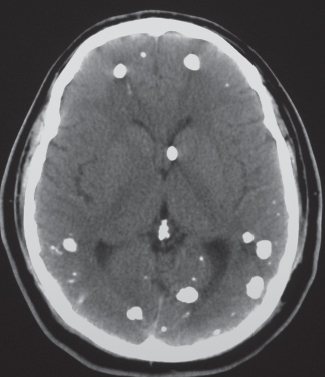
Metastases
Fig. 1.48a, b |
The CT appearance of a hemorrhagic metastatic lesion is one of an intracerebral hematoma involving a portion or all of the neoplasm, usually associated with peripheral edema (decreased attenuation), often multiple; with contrast enhancement in nonhemorrhagic portions of lesions. |
Metastatic intra-axial tumors associated with hemorrhage include bronchogenic carcinoma, renal cell carcinoma, melanoma, choriocarcinoma, and thyroid carcinoma. May be difficult to distinguish from hemorrhage related to other etiologies, such as vascular malformations and amyloid angiopathy. |
Vascular |
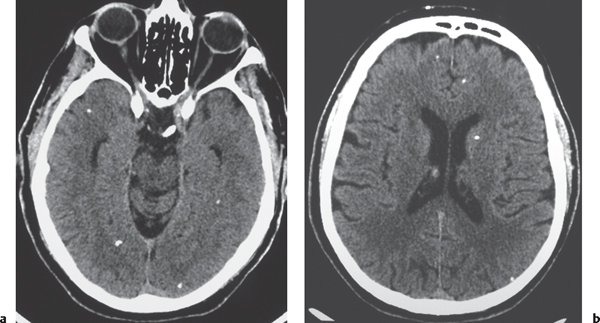
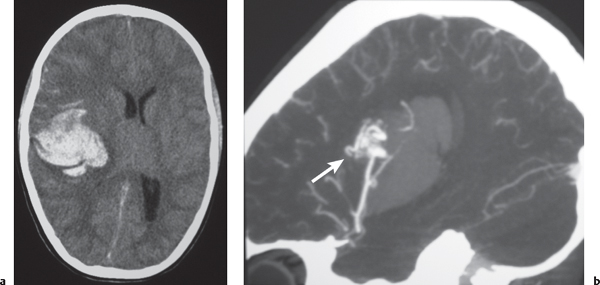
Arteriovenous malformation (AVM) |
Lesions with irregular margins that can be located in the brain parenchyma (pia, dura, or both locations).
CT: AVMs contain multiple tortuous tubular vessels that have intermediate or slightly increased attenuation that shows contrast enhancement. Calcifications occur in 30% of cases. Computed tomography angiography (CTA) shows arteries, veins, and nidus of AVM even when there is an intra-axial hemorrhage.
Fig. 1.49a, b
MRI: Serpiginous flow voids on T1- and T2-weighted images secondary to patent arteries with high blood flow, as well as thrombosed vessels with variable signal, areas of hemorrhage in various phases, calcifications, and gliosis. The venous portions often show gadolinium enhancement. Gradient echo MRI shows flow-related enhancement (high signal) in patent arteries and veins of the AVM. MRA using time of flight (TOF) or phase contrast techniques can provide additional detailed information about the nidus, feeding arteries and draining veins, and presence of associated aneurysms. Usually not associated with mass effect unless there is recent hemorrhage or venous occlusion. |
Supratentorial AVMs occur more frequently (80%–90%) than infratentorial AVMs (10%–20%). Annual risk of hemorrhage. AVMs can be sporadic, congenital, or associated with a history of trauma. Multiple AVMs can be seen in syndromes: Rendu-Osler-Weber (AVMs in brain and lungs, and mucosal, capillary telangiectasias); Wyburn-Mason (AVMs in brain and retina, with cutaneous nevi). |
Cavernous hemangioma |
Single or multiple multilobulated intra-axial lesions.
CT: Lesions have intermediate to slightly increased attenuation, with or without calcifications. |
Supratentorial cavernous angiomas occur more frequently than infratentorial lesions. Can be located in many different locations, multiple lesions > 50%. |
Venous angioma |
CT: No abnormality or small, slightly hyperdense zone prior to contrast administration. Contrast enhancement seen in a slightly prominent vein draining a collection of small veins.
MRI: On postcontrast T1-weighted images, venous angiomas are seen as a gadolinium contrast-enhancing transcortical vein draining a collection of small medullary veins (caput medusae). The draining vein can be seen as a signal void on T2-weighted images. |
Considered an anomalous venous formation typically not associated with hemorrhage; usually an incidental finding except when associated with cavernous hemangioma. |
Neuroepithelial cyst |
CT: Well-circumscribed cysts with low attenuation, no contrast enhancement.
MRI: Cysts have low signal on T1-weighted images and high signal on T2-weighted images, thin walls, no gadolinium contrast enhancement or peripheral edema. |
Cyst walls have histopathologic features similar to epithelium, neuroepithelial cysts; located in choroid plexus > choroidal fissure > ventricles > brain parenchyma. |
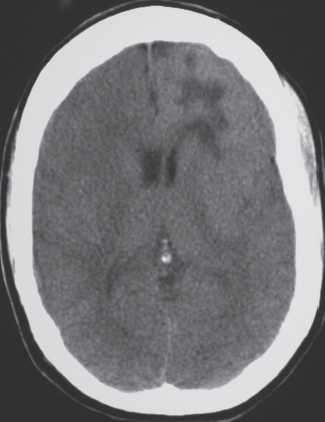
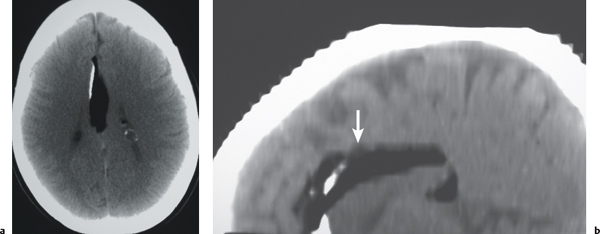
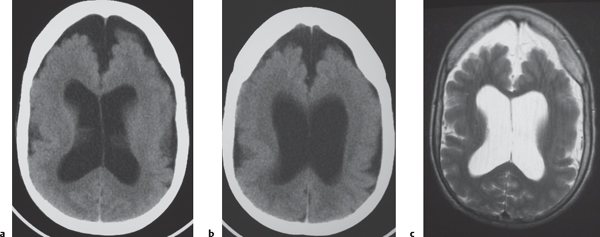
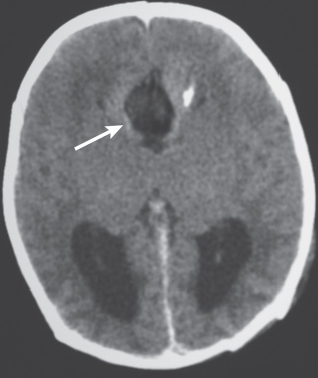
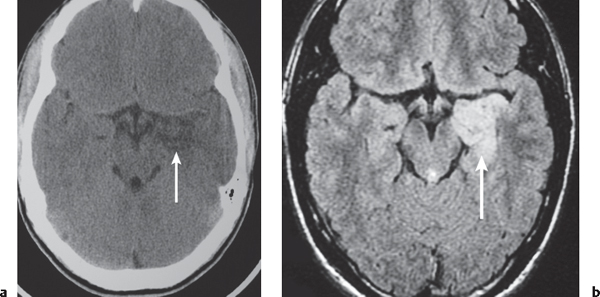
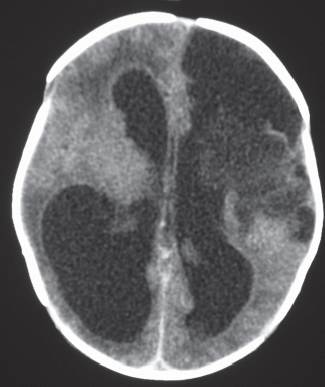
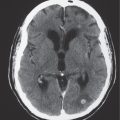
Porencephalic cyst
Fig. 1.50 |
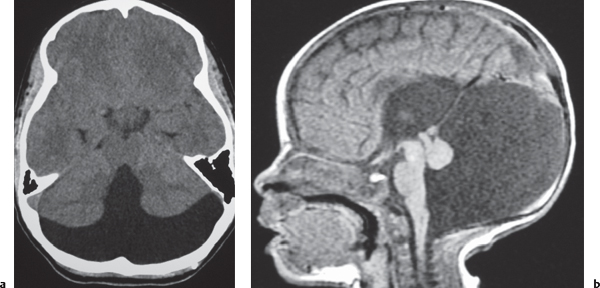
CT: Irregular, relatively well-circumscribed zone with low attenuation, no contrast enhancement.
MRI: Zone of low signal on T1-weighted images and high signal on T2-weighted images similar to CSF, surrounded by poorly defined thin zone of high T2 signal in adjacent brain tissue; no gadolinium contrast enhancement or peripheral edema. |
Cysts represent remote sites of brain injury (trauma, infarction, infection, or hemorrhage) with evolution into a cystic zone with CSF attenuation and MRI signal characteristics surrounded by gliosis in adjacent brain parenchyma. Gliosis (high T2 signal) allows differentiation from schizencephaly. |
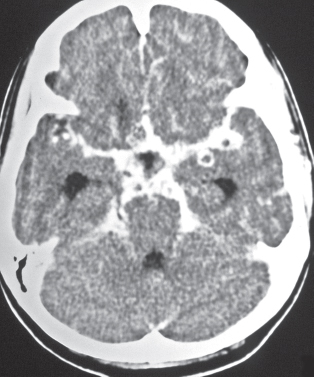
Cerebral infarction related to occlusion of large vessels |
CT and MRI features of cerebral and cerebellar infarcts depend on the age of the infarct relative to the time of examination.
Hyperacute (< 12 h):
CT: No abnormality (50%); decreased attenuation and blurring of lentiform nuclei; hyperdense artery up to 50%. MRI: Localized edema; usually isointense signal to normal brain on T1- and T2-weighted images. Diffusion-weighted images can show positive findings related to decreased apparent diffusion coefficients secondary to cytotoxic edema, absence of arterial flow void, or arterial enhancement in the vascular distribution of the infarct.
Acute (12–24 h):
CT: Zones with decreased attenuation in basal ganglia, blurring of junction between cerebral cortex and white matter, sulcal effacement.
MRI: Intermediate signal on T1-weighted images, high signal on T2-weighted images, localized edema. Signal abnormalities commonly involve the cerebral cortex and subcortical white matter and/or basal ganglia.
Early subacute (24 h–3 d):
CT: Localized swelling at sites with low attenuation involving gray and white matter (often wedge shaped), with or without hemorrhage MRI: Zones with low to intermediate signal on T1-weighted images, high signal on T2-weighted images, localized edema, with or without hemorrhage, with or without gadolinium contrast enhancement.
Late subacute (4 d–2 wk):
CT: Localized swelling increases and then decreases; low attenuation at lesion can become more prominent; with or without gyral contrast enhancement.
MRI: Low to intermediate signal on T1-weighted images, high signal on T2-weighted images, edema/mass effect diminishing, with or without hemorrhage, with or without contrast enhancement.
2 weeks to 2 months:
CT: with or without Gyral contrast enhancement, localized mass effect resolves.
MRI: Low to intermediate signal on T1-weighted images, high signal on T2-weighted images; edema resolves; with or without hemorrhage, with or without enhancement eventually declines. > 2 months:
CT: Zone of low attenuation associated with encephalomalacia.
MRI: Low signal on T1-weighted images, high signal on T2-weighted images, encephalomalacic changes, with or without calcification, hemosiderin.
Fig. 1.51a–d |
Cerebral infarcts usually result from occlusive vascular disease involving large, medium, or small arteries. Vascular occlusion may be secondary to atheromatous arterial disease, cardiogenic emboli, neoplastic encasement, hypercoagulable states, dissection, or congenital anomalies. Cerebral infarcts usually result from arterial occlusion involving specific vascular territories, although they occasionally occur from metabolic disorders (mitochondrial encephalopathies, etc.) or intracranial venous occlusion (thrombophlebitis, hypercoagulable states, dehydration, etc.), which do not correspond to arterial distributions. |