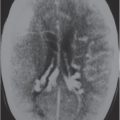
Normal variants |
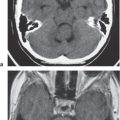
Choroid plexus calcifications
Fig. 1.196a, b |
Calcifications occur in the choroid plexus (lateral ventricles, atria, third and fourth ventricles, and foramina of Luschka). |
The choroid plexuses are invaginated folds of pia mater covered by cuboidal or low columnar epithelium of neural tube origin that extend into the ventricles. The epithelial cells of the choroid plexus secrete CSF into the ventricles. Normal physiologic calcifications in the choroid plexus begin in the pediatric population, 10% in the first 2 decades, and progressively increase in frequency with aging. |
Pineal calcification
Fig. 1.196a |
Incidental calcifications commonly occur in the pineal gland. |
Normal physiologic calcifications in the pineal gland occur in the pediatric population and progressively increase in frequency with aging. Calcifications usually measure < 1 cm in diameter. Can be seen in up to 40% of patients by age 20 y. |
Habenula
Fig. 1.196a |
Calcifications commonly occur in the habenula, which is located anterior to the pineal gland. |
The habenula is a small nuclear structure that is part of the epithalamus and is located anterior to the pineal gland. It receives input from the septal nucleus and thalamus from the stria medullaris and sends output to the interpeduncular nucleus. Normal physiologic calcifications in the habenula begin in the pediatric population and progressively increase in frequency with aging. |
Falcine/dural calcifications
Fig. 1.197a, b |
Calcifications can occur as incidental findings in various intracranial dural locations in adults. Dural calcifications in children may be associated with a dural tumor or genetic abnormality, such as basal cell nevus syndrome. |
The dura mater is the external layer of the meninges composed of dense connective tissue that is continuous with the inner periosteum of the skull. Metaplastic ossification changes in the dura commonly occur in adults. |
Arachnoid granulation |
Normal physiologic calcifications can be seen in the arachnoid granulations in adults, particularly within the transverse and sigmoid venous sinuses. |
Arachnoid granulations or villi are protrusions of the arachnoid (composed of connective tissue lacking blood vessels covered by squamous epithelium) into the dural venous sinuses. The function of the arachnoid granulations is to transfer CSF from the subarachnoid space into the blood of the venous sinuses. |
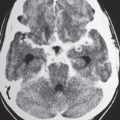
Idiopathic basal ganglia calcifications
Fig. 1.198 |
Punctate calcifications in the caudate nuclei, thalami, putamina, and/or globus pallidi bilaterally. |
The basal ganglia are involved in the initiation and modulation of movement. Physiologic and nonpatho-logic calcifications involving the caudate nuclei, putamen, and/or globus pallidus bilaterally occur in adults older than age 30 y. Occur in 1% to 2% of the population, usually in adults; increase in frequency with age. Idiopathic calcifications account for 75% of basal ganglia calcifications. Calcifications in these locations can also occur with disorders of calcium and phosphate metabolism (hypoparathyroidism, pseudohypoparathyroidism, pseudo-pseudohypoparathyroidism, hyperparathyroidism, and carbonic anhydrase II deficiency). |
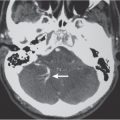
Dentate nuclei calcifications
Fig. 1.199 |
Calcifications in the dentate nuclei of the cerebellar hemispheres. |
The dentate nuclei are the most lateral of the deep cerebellar nuclei. They receive input from the lateral cerebellar hemispheres and send output via the superior cerebellar peduncles. Physiologic and nonpatho-logic calcifications involving the dentate nuclei occur in adults usually older than age 30 y. |
Vascular |
Atherosclerotic calcifications
Fig. 1.200 |
Calcified and/or soft atherosclerotic plaques in the walls of arteries in adults. Common intracranial sites include the cavernous and supraclinoid portions of the internal carotid arteries and upper vertebral arteries and basilar artery. |
Commonly seen with older adults secondary to athero-sclerotic arterial disease. |
AVM
Fig. 1.201a, b |
Lesions with irregular margins that can be located in the brain parenchyma or meninges (pia, dura, or both locations). AVMs contain multiple tortuous enhancing blood vessels secondary to patent arteries with high blood flow, as well as thrombosed vessels with variable attenuation, areas of hemorrhage in various phases, calcifications, and gliosis. The venous portions often show contrast enhancement. CTA can provide additional detailed information about the nidus, feeding arteries, and draining veins, as well as the presence of associated aneurysms. Usually not associated with mass effect unless there is recent hemorrhage or venous occlusion. |
Supratentorial AVMs occur more frequently (80%–90%) than infratentorial AVMs (10%–20%). Annual risk of hemorrhage. AVMs can be sporadic, congenital, or associated with a history of trauma. Multiple AVMs can be seen in syndromes: Rendu-Osler-Weber (AVMs in brain and lungs and mucosal capillary telangiectasias) and Wyburn-Mason (AVMs in brain and retina with cutaneous nevi). |
Cavernous angioma
Fig. 1.202 |
Single or multiple multilobulated intra-axial lesions that have intermediate to slightly increased attenuation; minimal or no contrast enhancement; with or without calcifications. |
Cavernous angiomas can be located in many different locations; multiple lesions > 50%. Associated with venous angiomas and risk of hemorrhage. |
Giant aneurysm
Fig. 1.203 |
Focal, well-circumscribed structure with layers of low, intermediate, and/or high attenuation secondary to layers of thrombus of different ages, as well a zone of contrast enhancement representing a patent lumen if present. With or without wall calcifications. |
Saccular aneurysms > 2.5 cm in diameter are referred to as giant aneurysms. Fusiform aneurysms are often related to atherosclerosis or collagen vascular disease (Marfan syndrome, Ehlers-Danlos syndrome, etc.). Dissecting aneurysms: hemorrhage occurs in the arterial wall from incidental or significant trauma. |
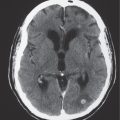
Dystrophic calcifications
Fig. 1.204 |
Dystrophic calcifications can occur at sites of prior cerebral and cerebellar infarction, intracerebral and extra-axial hematomas, and radiation treatment, as well as from chemotherapy. |
Dystrophic calcifications result from mineralizing microangiopathy involving small arteries and arterioles causing calcifications in the basal ganglia and subcortical white matter. Necrotizing leukoencephalopathy is another cause of dystrophic calcifications in white matter. |
Sturge-Weber syndrome
Fig. 1.205a–c |
Prominent localized unilateral leptomeningeal enhancement usually in parietal and/or occipital regions in children; with or without gyral enhancement; mild localized atrophic changes in brain adjacent to the pial angioma; with or without prominent medullary and/or subependymal veins; with or without ipsilateral prominence of choroid plexus. Gyral calcifications > 2 y, progressive cerebral atrophy in region of pial angioma. |
Also known as encephalotrigeminal angiomatosis; neurocutaneous syndrome associated with ipsilateral “port wine” cutaneous lesion and seizures; results from persistence of primitive leptomeningeal venous drainage (pial angioma) and developmental lack of normal cortical veins producing chronic venous congestion and ischemia. |
Inflammation/infection |
Parasites (toxoplasmosis, cysticercosis, hydatid, Paragonimus), viruses (CMV, herpes), syphilis, and tuberculosis |
|
|
Toxoplasmosis
Fig. 1.206 |
Single or multiple solid and/or cystic-appearing lesions located in basal ganglia and/or corticomedullary junctions in cerebral hemispheres; low to intermediate attenuation, nodular or rim pattern of contrast enhancement, with or without mild peripheral low attenuation (edema).
Chronic phase: Calcified granulomas. |
Most common opportunistic CNS infection in AIDS patients, caused by ingestion of food contaminated with parasites (Toxoplasma gondii). Can also occur as congenital or neonatal infection (TORCH: Toxoplasma, rubella, CMV, herpes). |
Cysticercosis
Fig. 1.207a–c |
Single or multiple cystic-appearing lesions in brain or meninges.
Acute/subacute phase: Low to intermediate attenuation, rim with or without nodular pattern of contrast enhancement, with or without peripheral low attenuation (edema).
Chronic phase: Calcified granulomas. |
Caused by ingestion of ova (Taenia solium) in contaminated food (undercooked pork); involves meninges > brain parenchyma > ventricles. |
Hydatid cyst |
Echinococcus granulosus: Single or rarely multiple cystic-appearing lesions with low attenuation surrounded by a thin wall; typically no contrast enhancement or peripheral edema unless superinfected; often located in the vascular territory of the middle cerebral artery.
Echinococcus multilocularis: Cystic (with or without multilocular) and/or solid lesions; central zone of intermediate attenuation surrounded by a slightly thickened rim, with contrast enhancement. Peripheral zone of decreased attenuation (edema) and calcifications are common. |
Caused by parasites E. granulosus (South America, Middle East, Australia, and New Zealand) and E. multilocularis (North America, Europe, Turkey, and China). CNS involvement in 2% of cases of hydatid infestation. |
Benign neoplasms |
Meningioma
Fig. 1.208a, b |
Extra-axial dural-based lesions, well-circumscribed; supratentorial > infratentorial, parasagittal > convexity > sphenoid ridge > parasellar > posterior fossa > optic nerve sheath > intraventricular; intermediate attenuation, usually prominent contrast enhancement; with or without calcifications, with or without hyperostosis of adjacent bone. |
Most common extra-axial tumor, usually benign neoplasms, typically occurs in adults (older than 40 y), women > men; multiple meningiomas seen with neurofibromatosis type II. Can result in compression of adjacent brain parenchyma, encasement of arteries, and compression of dural venous sinuses; rarely invasive/malignant types. |
Lipoma of the corpus callosum
Fig. 1.209a, b |
Lipomas have CT attenuation similar to subcutaneous fat, with or without calcifications; typically no contrast enhancement or peripheral edema. |
Benign fatty lesions resulting from congenital malformation often located in or near the midline; may contain calcifications and/or traversing blood vessels; may be associated with dysplasia/hypoplasia of the corpus callosum. |
Choroid plexus papilloma |
Circumscribed and/or lobulated lesions with papillary projections, intermediate attenuation, usually prominent contrast enhancement, with or without calcifications. Locations: atrium of lateral ventricle (children) > fourth ventricle (adults), rarely other locations, such as third ventricle. Associated with hydrocephalus. |
Rare intracranial neoplasms, choroid plexus papillomas may rarely disseminate along CSF pathways. |
Central neurocytoma |
Circumscribed lesion located at the margin of the lateral ventricle or septum pellucidum with intraventricular protrusion, heterogeneous low and intermediate attenuation, with or without calcifications and/or small cysts; heterogeneous contrast enhancement. |
Rare tumors that have neuronal differentiation, imaging appearance similar to intraventricular oligodendrogliomas; occur in young adults; benign, slow-growing lesions. |
Ganglioglioma/ganglioneuroma |
Circumscribed tumor, usually supratentorial; often temporal or frontal lobes; low to intermediate attenuation; with or without cysts, with or without calcifications, with or without contrast enhancement. |
Ganglioglioma (contains glial and neuronal elements), ganglioneuroma (contains only ganglion cells). Uncommon tumors, < 30 y, seizure presentation, slow-growing neoplasms. Gangliocytoma (contains only neuronal elements, dysplastic brain tissue). Favorable prognosis if completely resected. |
Craniopharyngioma
Fig. 1.210a–c |
Circumscribed lobulated lesions; both suprasellar and intrasellar location > suprasellar > intrasellar; variable low, intermediate, and/or high attenuation; with or without nodular or rim contrast enhancement. May contain cysts, lipid components, and calcifications. |
Usually histologically benign but locally aggressive lesions arising from squamous epithelial rests along the Rathke cleft; occurs in children (peak range, 5–15 y) and adults (> 40 y), males = females. |
Osteoma |
Well-circumscribed lesions involving the skull with high attenuation similar to cortical bone; typically show no contrast enhancement. |
Benign proliferation of dense bone located in the skull or paranasal sinuses (frontal > ethmoid > maxillary > sphenoid). |
Tuberous sclerosis
Fig. 1.211 |
Cortical/subcortical lesion with variable attenuation: Calcifications in 50% of older children; contrast enhancement uncommon.
Subependymal hamartomas: Small nodules located along and projecting into the lateral ventricles; calcifications and contrast enhancement are common. |
Cortical and subependymal hamartomas are nonmalignant lesions associated with tuberous sclerosis. Tuberous sclerosis is an autosomal dominant disorder associated with hamartomas in multiple organs. |
Giant cell astrocytoma/tuberous sclerosis
Fig. 1.212a, b |
Circumscribed lesion located near the foramen of Monro with mixed low to intermediate attenuation, with or without cysts and/or calcifications, with heterogeneous contrast enhancement. |
Subependymal hamartoma near the foramen of Monro; occurs in 15% of patients with tuberous sclerosis younger than 20 y; slow-growing lesions that can progressively cause obstruction of CSF flow through the foramen of Monro; long-term survival usual if resected. |
Malignant tumors |
Metastatic disease |
Circumscribed spheroid lesions in brain; can have various intra-axial locations, often at gray-white matter junctions; usually low to intermediate attenuation; with or without hemorrhage, calcifications, cysts; variable contrast enhancement, often associated with adjacent low attenuation from axonal edema. |
Represent ~33% of intracranial tumors, usually from extracranial primary neoplasm in adults older than 40 y. Primary tumor source: lung > breast > GI > GU > melanoma. Metastatic lesions associated with calcifications include osteosarcoma, mucinous adeno-carcinoma, and renal cell carcinoma. |
Oligodendroglioma
Fig. 1.213 |
Circumscribed lesion with mixed low to intermediate attenuation; sites of clumplike calcification; heterogeneous contrast enhancement; involves white matter and cerebral cortex; can cause chronic erosion of the inner table of the calvarium. |
Uncommon slow-growing gliomas with usually mixed histologic patterns (astrocytomas, etc.). Usually seen in adults older than 35 y; 85% supratentorial. If low grade, 75% 5-y survival; higher grade lesions have a worse prognosis. |
Ependymoma
Fig. 1.214a, b |
Circumscribed lobulated supratentorial lesion, often extraventricular; with or without cysts and/or calcifications; low to intermediate attenuation; variable contrast enhancement. |
Tumors occur more commonly in children than adults; one third supratentorial, two thirds infratentorial; 45% 5-y survival. |
Primitive neuroectodermal tumors
Fig. 1.215 |
Circumscribed or invasive lesions, low to intermediate attenuation; variable contrast enhancement, with or without cysts, with or without calcifications; frequent dissemination into the leptomeninges. |
Highly malignant tumors that frequently disseminate along CSF pathways. |
Atypical teratoid/rhabdoid tumor
Fig. 1.216a, b |
Circumscribed or poorly defined mass lesions with intermediate attenuation, with or without zones of high attenuation from hemorrhage; usually prominent contrast enhancement with or without heterogeneous pattern. |
Rare malignant tumors involving the CNS usually occurring in the first decade. Histologically appear as solid tumors with or without necrotic areas; similar to malignant rhabdoid tumors of the kidney. Associated with a poor prognosis. |
Choroid plexus carcinoma
Fig. 1.217a, b |
Circumscribed and/or lobulated lesions with papillary projections; intermediate attenuation, usually prominent contrast enhancement, with or without calcifications. Locations: atrium of lateral ventricle (children) > fourth ventricle (adults); rarely other locations, such as third ventricle; associated with hydrocephalus. |
Rare intracranial neoplasms, choroid plexus carcinomas frequently disseminate along CSF pathways and invade brain tissue. Carcinomas are often larger than papillomas. Carcinomas may have heterogeneous mixed attenuation, with or without hemorrhage, with or without calcifications, with or without brain invasion. |
Hemangiopericytoma |
Extra-axial mass lesions, often well circumscribed, intermediate attenuation, prominent contrast enhancement (may resemble meningiomas), with or without associated erosive bone changes, with or without calcifications. |
Rare neoplasms in young adults (men > women) sometimes referred to as angioblastic meningioma or meningeal hemangiopericytoma; arise from vascular cells and pericytes; frequency of metastases > meningiomas. |
Malignant meningioma |
Extra-axial dural-based lesions, supratentorial > infratentorial; heterogeneous mixed attenuation, usually prominent heterogeneous contrast enhancement, irregular margins with invasion of adjacent brain, with or without calcifications, with or without hyperostosis of adjacent bone. |
Extra-axial tumor that typically occurs in adults older than 40 y, women > men; occasionally occurs in children. Multiple meningiomas seen with neurofibromatosis type 2; can result in compression of adjacent brain parenchyma, encasement of arteries, and compression of dural venous sinuses. Malignant meningiomas often invade adjacent tissues. |
Teratoma
Fig. 1.218a, b |
Circumscribed lesions; pineal region > suprasellar region > third ventricle; variable low, intermediate, and/or high attenuation; with or without contrast enhancement. May contain calcifications, as well as fatty components, which can cause chemical meningitis if ruptured. |
Second most common type of germ cell tumor; occurs in children, males > females; benign or malignant types; composed of derivatives of ectoderm, mesoderm, and/or endoderm. |
Pineal gland tumors
Fig. 1.219a, b |
Tumors often have intermediate attenuation to intermediate to slightly high attenuation, with contrast enhancement, with or without central and/or peripheral calcifications. Malignant tumors are often larger than benign pineal lesions (pineocytoma), as well as heterogeneous attenuation and contrast enhancement pattern. With or without leptomeningeal tumor. |
Pineal gland tumors account for 8% of intracranial tumors in children and 1% of tumors in adults; 40% of tumors are germinomas, followed by pineoblastomas and pineocytomas, teratomas, choriocarcinomas, endodermal sinus tumors, astrocytomas, and metastatic tumors. |
Chordoma |
Well-circumscribed, lobulated lesions destroying bone along the dorsal surface of the clivus, vertebral bodies, or sacrum.
CT: Low to intermediate attenuation, with or without calcifications from destroyed bone carried away by tumor, with contrast enhancement.
MRI: Lesions have low to intermediate signal on T1-weighted images, high signal on T2-weighted images, with gadolinium enhancement (usually heterogeneous); locally invasive associated with bone erosion/destruction, encasement of vessels and nerves; skull base/clivus common location, usually in the midline. |
Rare, slow-growing, destructive tumors derived from notochordal remnants; detailed anatomical display of extension of chordomas by CT and MRI is important for planning of surgical approaches. |
Chondrosarcoma
Fig. 1.220 |
Lobulated lesions, low to intermediate attenuation, with or without chondroid matrix mineralization, with contrast enhancement (usually heterogeneous); locally invasive associated with bone erosion/destruction, encasement of vessels and nerves, skull base petrooccipital synchondrosis common location, usually off midline. |
Rare, slow-growing, malignant cartilaginous tumors; detailed anatomical display of extension of chondrosarcomas by CT and MRI is important for planning of surgical approaches. |
Osteosarcoma
Fig. 1.221 |
Destructive lesions involving the skull base, low to intermediate attenuation, usually with matrix mineralization/ossification, with contrast enhancement (usually heterogeneous). |
Rare lesions involving the endochondral bone-forming portions of the skull base or membranous-bone forming calcarium; more common than chondrosarcomas and Ewing sarcoma; locally invasive, high metastatic potential. Occurs in children as primary tumors and adults (associated with Paget disease, irradiated bone, chronic osteomyelitis, osteoblastoma, giant cell tumor, and fibrous dysplasia). |
Metabolic/idiopathic |
Hypothyroidism |
Punctate calcifications in the caudate nuclei, thalami, putamina, and/or globus pallidi bilaterally. |
Disorder from insufficient amount of thyroid hormone that can be congenital; associated with developmental abnormalities (cretinism) or from loss of thyroid tissue (autoimmune diseases that produce antithyroid antibodies or antibodies that block the thyroid-stimulating hormone [TSH] receptor, surgery, or radioiodine ablation for treatment of Graves disease). |
Hyperparathyroidism |
Punctate calcifications in the caudate nuclei, thalami, putamina, and/or globus pallidi bilaterally. |
Primary type results from excess production of parathyroid hormone (PTH) from hyperplasia or adenomas involving the parathyroid gland (s). PTH regulates calcium and phosphate blood levels. Excessive PTH secretion results in hypercalcemia and hypophosphatemia. Secondary hyperparathyroidism occurs with elevated PTH levels in response to low calcium levels from disorders such as chronic renal disease and vitamin D deficiency. Calcium levels in blood are low or normal, and phosphate levels are usually elevated. |
Hypoparathyroidism |
Punctate calcifications in the caudate nuclei, thalami, putamina, and/or globus pallidi bilaterally. |
Hypoparathyroidism occurs when there is a deficiency in formation of PTH, which regulates metabolism of calcium, phosphorus, and vitamin D. Deficiency of PTH from the parathyroid glands results in decreased blood calcium levels and elevated blood phosphorus levels. Can result from injury to the parathyroid glands during head and neck surgery or radioactive iodine treatment for hyperthyroidism. DiGeorge syndrome of hypoparathyroidism occurs because of congenital absence of the parathyroid glands. Familial hypoparathyroidism occurs with other endocrine diseases, such as adrenal insufficiency, in a syndrome called type I polyglandular autoimmune syndrome. |
Pseudohypoparathyroidism |
Punctate calcifications in the caudate nuclei, thalami, putamina, and/or globus pallidi bilaterally |
Pseudohypoparathyroidism is a rare syndrome in which there is resistance to the effects of PTH in the body resulting in low blood calcium levels and high blood phosphate levels. Associated with dysfunctional G proteins: Gs–a subunit, mutation in GNAS1 gene. PTH levels are often elevated. Type Ia is autosomal dominant and results in short stature, round face, and short fourth and fifth metacarpal bones; is also called Albright hereditary osteodystrophy. Associated with abnormal GNAS1 gene. Type Ib involves resistance to PTH only in the kidneys and is associated with a methylation defect; lacks the physical features of type I. Type II is very similar to type I, but the events that take place in the kidneys are different. Type II pseudohypoparathyroidism is associated with low blood calcium and high blood phosphate levels, although there is absence of the physical characteristics associated with type I. |
Pseudo-pseudohypoparathyroidism (pseudo-PHP) |
Punctate calcifications in the caudate nuclei, thalami, putamina, and/or globus pallidi bilaterally |
Inherited disorder with symptoms and phenotypic appearance of pseudohypoparathyroidism type 1. Caused by a defect on the corresponding maternal chromosome as pseudo-PHP 1. Pseudo-PHP presents only with the skeletal defects of PHP, although patients have normal blood levels of calcium, phosphate, and PTH. |
MELAS and MERRF syndromes |
CT: Symmetric zones of low attenuation in the basal ganglia, along with cerebral infarction that is not limited to one vascular distribution. With or without dystrophic calcifications in basal ganglia. |
MELAS is a maternally inherited disease affecting transfer RNA in mitochondria. |
|
MRI: High T2 signal in basal ganglia usually symmetric; high T2 signal in cerebral and cerebellar cortex and subcortical white matter not corresponding to a specific large arterial vascular territory. Signal abnormalities may resolve and reappear. |
MERRF is a mitochondrial encephalopathy associated with muscle weakness and myoclonic epilepsy, short stature, ophthalmoplegia, and cardiac disease. |
Fahr disease
Fig. 1.222a–d |
Intra-axial calcifications occur in the basal ganglia, dentate nuclei, and cerebral white matter. |
Fahr disease, also known as familial cerebrovascular ferrocalcinosis, is a group of disorders with deposition of calcification in the brain. |
Cockayne syndrome
Fig. 1.223a–c |
Calcifications occur in the basal ganglia and dentate nuclei. Cerebral and cerebellar atrophy is also seen. |
Autosomal recessive disease in children with cutaneous photosensitivity, progressive neurologic impairment, optic atrophy, cataracts, dwarfism, and thoracic kyphosis. |
Neuronal ceroid lipofuscinosis |
Progressive cerebral and cerebellar atrophy with or without calcifications in the brain. |
Inherited progressive neurodegenerative disorders consisting of multiple types: infantile, late infantile, juvenile (Batten disease), early juvenile, adult dominant, adult recessive, and progressive epilepsy with mental retardation. The different types have similar clinical features of visual dysfunction, seizures, impairment of speech and motor function, and progressive dementia. |