10 Functional MRI and Diffusion Tensor Imaging with Tractography
10.1 Introduction
Surgery is an effective and potentially curative treatment for many patients with brain tumors, with durable improvements in the length and quality of patient survival as well as the efficacy of adjuvant treatments after gross total resection.1,2,3,4,5,6 Although surgery should maximize tumor resection, the overarching goal remains patient safety and avoiding inadvertent injury to eloquent brain that may cause profound neurologic deficits. Direct intraoperative mapping techniques remain the reference standards for mapping eloquent cortex and white matter. These are invasive, time-consuming techniques, however, which require a large craniotomy, exposure of the area of interest, and possibly an awake surgery with the risk of seizure or neurologic worsening.
Functional magnetic resonance imaging (fMRI) and diffusion tensor imaging (DTI) with tractography are complementary functional imaging techniques for the noninvasive evaluation and mapping of eloquent brain areas. Active clinical indications for fMRI and DTI are summarized in box Clinical applications of functional magnetic resonance imaging and diffusion tensor imaging. Potential benefits include shortening the time of the operation spent mapping eloquent areas, maximizing resection of the tumor, and avoiding potentially devastating neurologic injuries. The two most common applications in brain tumor patients are for preoperative mapping of sensorimotor and language cortices and white matter tracts. This chapter reviews the basic principles of fMRI, DTI, and tractography with an emphasis on their applications, challenges, and limitations for clinical care.
Clinical applications of functional magnetic resonance imaging and diffusion tensor imaging
To guide clinical decision making about whether to perform a resection and the planned extent of the resection, vs. a stereotactic biopsy or not to operate
To assist patient counseling about the feasibility, risks, and benefits of surgery
To determine the safest approach to reach the tumor
To guide intraoperative stimulation that is performed to confirm the imaging results rather than mapping de novo
To guide the procedure if intraoperative stimulation fails or the area in question is surgically inaccessible
10.2 fMRI
10.2.1 BOLD fMRI
Blood oxygenation level dependent (BOLD) fMRI indirectly measures changes in brain activation through the mechanism of neurovascular coupling. Increased neuronal activity leads to a predictable hemodynamic response where changes in the oxygenation state of hemoglobin can be measured as small alterations in the local magnetic environment. Oxyhemoglobin has diamagnetic properties with relatively small susceptibility and long T2* effects. In contrast, the four unpaired electrons of deoxyhemoglobin impart paramagnetic properties with relatively large susceptibility and short T2* effects. These local hemodynamic changes form the basis of BOLD imaging to estimate and localize areas of neuronal activation.
Local cortical activation initially consumes oxyhemoglobin, increasing the relative fraction of deoxyhemoglobin in blood and causing a tiny decrease in the BOLD signal that is difficult to detect.7 The neuronal activity also causes rapid vasodilatation with increased cerebral blood flow (CBF), cerebral blood volume (CBV), and delivery of oxyhemoglobin. The increase in CBF and subsequent increase in oxyhemoglobin overshoot the increase in local cerebral metabolic rate of oxygen demand. Therefore, net increased BOLD signal is observed from the increased relative fraction of diamagnetic oxyhemoglobin, despite increased oxygen demand and extraction.8 The net decrease in paramagnetic deoxyhemoglobin results in increased signal on the T2* images, which correlates to the site of the neuronal activity. The increased BOLD signal then decays and may undershoot as it returns to baseline. The magnitude of these BOLD changes is on the order of 2 to 4%.
Clinical BOLD fMRI is most commonly acquired with gradient echo (GE) echoplanar imaging (EPI), which permits whole brain coverage with high spatial resolution (< 3 mm) and high temporal resolution (2–3 s). Although the spatial resolution is adequate to localize activation signal to a gyrus, the functional data are routinely coregistered onto higher-resolution and higher-contrast anatomical images for interpretation. GE EPI is highly sensitive to changes in T2* signal resulting from increased magnetic susceptibility caused by deoxyhemoglobin. Contributions to T2* signal occur mostly from voxels containing the capillary and venous beds, however, indicating that mapping of the functional activity may be displaced toward the venous drainage and not at the actual site of cortical activation. fMRI may also be acquired with spin-echo (SE) EPI, which measures only the capillary BOLD signal. Despite a smaller measurable signal, and therefore lower contrast between active and rest phases, SE EPI generates smaller measureable signals and therefore lower contrast between active and rest phases. Despite these disadvantages, SE EPI has superior resolution to localize submm functional areas in eloquent cortex, and also performs better when susceptibility artifacts are prominent (e.g., due to prior surgery, metal, hemorrhage, air-bone interfaces).
Generation of activation maps requires several data analysis steps: preprocessing, regression, and inference. First, the raw images are preprocessed to temporally interpolate images obtained at different times, and spatially registered to correct for head motion. Spatial smoothing may be used to augment the signal-to-noise ratio, and spatial normalization to a standard stereotactic space to enable group analyses. Second, the paradigm delivery is convolved with the hemodynamic response function estimate, and the subsequent general linear model fitted to generate a map of regression coefficients. Third, the regression coefficients are statistically translated into z scores or p-values to generate activation maps, which are color scaled to the magnitude above the statistical significance level.
10.2.2 Neurologic Planning with fMRI
fMRI has an important role in the neurosurgical planning and the treatment counseling of patients with brain tumors. Determining the location of eloquent brain and its relationship with the brain tumor allows the neurosurgeon to determine the safest approach for the resection, and to provide accurate assessments of the expected outcomes and risks involved. This information would not be available with more traditional, invasive intraoperative mapping techniques. Paradigms and their utilities are discussed in detail next.
Paradigm Design
Clinical fMRI is usually performed using block design paradigms, where stimulus presentations and/or active states are regularly alternated with rest states over a 3 to 4 min scan. Most default paradigms usually consist of 30 s active and 30 s rest states that are repeated four to six times. We perform 20 s active and 40 s rest states to permit more complete return of the hemodynamic response to baseline. The rest state may consist of no activity, or an activity similar to that in the active state minus the function being tested. Block design paradigms are more sensitive for detecting differences between active and rest states than event-related paradigms, which present a single event stimulus over the entire scan duration rather than multiple epochs of stimuli. The major disadvantage of the event-related paradigms is the long interstimulus interval (~ 20 s) that often renders its use impractical. Paradigm selection is primarily determined by lesion location using information obtained by prior anatomical imaging, and must incorporate information about the patient’s ability to perform the paradigms and preexisting neurologic deficits. Most brain tumor cases involve sensorimotor and language paradigms, although fMRI may also be used to localize auditory, visual, and memory areas.
Sensorimotor Paradigms
Sensorimotor paradigms are used to localize the primary motor cortex (M1) in the precentral gyrus and to identify the central sulcus. Paradigm selection is based on patient presentation and tumor location relative to the motor homunculus. From medial to lateral, the motor homunculus is organized with areas for legs, hands, fingers, lips, and tongue (Fig. 10.1).

Foot motor paradigms include toe wiggling and foot dorsiflexion/plantarflexion (i.e., step on gas, lift up off gas). Somatomotor activation occurs in the paramedian precentral gyrus, paracentral lobule, and central sulcus. Visualization may be confounded by rich venous drainage from paramedian cortical veins into the superior sagittal sinus at this level. Somatosensory cortex, premotor, and cerebellar activation may also occur.
Hand motor paradigms usually consist of finger–thumb tapping or hand grasping. Due to the disproportionately large hand representation along the omega portion of the motor cortex, hand paradigms should be performed in all patients undergoing motor fMRI. Finger tapping results in activation in the omega portion of the primary somatomotor cortex as well as the supplementary motor area (SMA), sensory cortex, premotor cortex, and cerebellum.
Two common face motor paradigms are puckering or pouting for lips, and closed mouth tongue motions. Activation occurs in the lateral portion of the precentral gyrus assigned to the lower face, as well as in the sensory cortex, SMA, and premotor cortex. Tongue motions activation occurs slightly more laterally in the primary sensorimotor cortex and is often associated with cerebellar activation.
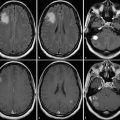
Bilateral paradigms should be used to gain information from the (usually) normal contralateral side, which can aid localization inferences for the tumor side when suboptimal results occur. In addition, paradigms can be combined (e.g., hand and foot) in cooperative patients in order to save separate scan time for each paradigm (Fig. 10.2). Because similar activation patterns are observed for similar motor paradigms, the exact paradigm chosen is usually not important.9 Paradigms that require motor planning, such as sequential finger thumb tapping, occasionally result in exuberant activation of premotor, motor, and sensory areas that may obscure the localization of the primary motor cortex. In completely paralyzed patients, passive movements may be performed to elicit BOLD motor activation. Active movements have been described as yielding more but not consistently better results.10 Purely sensory paradigms (e.g., stroking the hand and/or foot) may also be performed to localize the primary somatosensory cortex (S1), in order to deduce the central sulcus and location of the primary somatomotor cortex (Fig. 10.3 and Fig. 10.4).



Motor Mapping
The primary goal of motor fMRI is to localize the primary sensorimotor cortex by identifying the precentral or motor gyrus and the central sulcus. Activation occurs on the posterior cortex of the motor gyrus, or may occasionally follow the venous drainage and be displaced in a posterior direction onto the central sulcus. The motor homunculus is organized, from medial to lateral, with cortical representations for the foot, leg, hand, trunk, face, and tongue. Many neurosurgeons consider the foot area to be the most important, with an inability to ambulate imposing greater morbidity than asymmetry of the face or an inability to use one hand. The foot motor area is also the most difficult portion of the homunculus to identify at intraoperative stimulation, due to its paramedian location deep to cortical veins converging on the superior sagittal sinus, and its deep extension down into the posterior interhemispheric fissure away from the brain surface.
Language Paradigms
Language paradigm selection has the additional complexity of patient handedness, diversity of primary and/or secondary language centers, and potential multilingualism. Formal evaluation of handedness using the Edinburgh inventory11,12 should be performed if there is any doubt about hand dominance or ambidexterity. The implications of handedness on language lateralization are summarized in Table 10.1.
Handedness | Lesion location | Aphasia | fMRI |
Right | Right | Yes | Yes |
Left | Any | Yes | |
Left | Right | Any | Yes |
Left | Any | Yes | |
Ambidextrous | Right | Any | Yes |
Left | Any | Yes |
The Broca and Wernicke areas are widely considered primary, essential language centers. In contradistinction to the motor cortex, no reliable anatomical landmarks are available to localize functional language areas. The Broca area is classically defined in the pars opercularis and pars triangularis of the inferior frontal gyrus (Brodmann area 44, 45). Damage may result in expressive language disorders such as dysarthria, nonfluent speech, semantic and phonemic paraphasias, and/or mutism. The Wernicke area is classically defined in the posterior superior temporal gyrus (Brodmann area 22), and/or in the middle temporal gyrus (Brodmann area 21), supramarginal gyrus (Brodmann area 40), and angular gyrus (Brodmann area 39). Damage may cause comprehension language disorders such as fluent aphasia, semantic and phonemic paraphasias, circumlocutions, and/or word-finding difficulties.
Speech paradigms may be performed silently (covertly) or vocalized (overtly). Silent language paradigms provide robust and reproducible language localization and lateralization that is comparable to vocalized language paradigms, although the magnitude of activation may be less.13,14 Silent paradigms may also not correlate as well with sites of speech arrest at intraoperative stimulation as vocalized speech paradigms.15 Language lateralization may be less robust with vocalized paradigms, however, due to confounding by increased bilateral motor activation.14 We usually perform two to three speech paradigms in each patient, one of which will be vocalized with specific instructions to avoid head motion. Although uncommonly used, event-related designs may be applicable for vocalized speech paradigms intended to provide localization maps rather than lateralization.
Frontal language testing includes word-generation paradigms, verb-to-noun-generation paradigms, and object-naming paradigms. These require semantic processing to retrieve the concept from prior knowledge, phonological processing to retrieve a sound representation of the target, verbal working memory to store and manipulate the word-related data, and lexical search systems. The paradigms usually activate the Broca area, inferior frontal gyrus, and premotor frontal areas as well as the Wernicke area.13,16 Common word-generation paradigms include phonemic fluency paradigms (e.g., name words that begin with R or M, or generate an antonym to a presented word) and semantic fluency paradigms from a given lexical category (e.g., names of fruits and vegetables) (Fig. 10.5). Word-generation paradigms may generate less activation than sentence-reading paradigms in patients with aphasia.17 Object naming (e.g., name objects presented visually on a monitor or goggles) is a common paradigm to localize the inferior frontal and posterior parietal temporal speech and language regions, including the dorsolateral prefrontal cortex, inferior frontal gyrus, cingulate, SMA, premotor, and motor regions. Rest or control states may consist of rest or fixation on nonsense symbols. Occasionally, rest may involve reading or repeating matched control stimuli, which enable subtraction of semantic processing and phonological processing areas to better isolate working memory and lexical search areas in the inferior frontal gyrus.18

Posterior language testing includes forced choice reading and sentence completion paradigms (e.g., George cuts and washes other people’s hair. He is a _______), semantic fluency paradigms (e.g., names of fruits or animals), and auditory response naming paradigms (e.g., What color is grass? What do you brush your hair with?). Sentence generation, processing, and semantic decision paradigms often lead to stronger activation in the Wernicke area than the single-word processing involved in word-generation and picture-naming paradigms.19 The sentence and reading paradigms may also be more successful in eliciting language activation in aphasic patients.17 In contrast to the sentence-reading paradigms that involve reproduction and production of only one appropriate word, success of the word-generation paradigms relies on the production of many words within the active state and may be suboptimal with production of only a few words. Rhyming paradigms may also be used. During the active state, the patient is shown pairs of words for 3 to 5 seconds and requested to press a button if the words rhyme. In the rest state, two rows of sticks are shown for 3 to 5 seconds and the patient is requested to press a button if the two rows match.
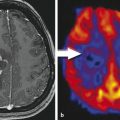
In severely aphasic patients unable to perform these paradigms, passive listening to vocalized speech or reading of visually presented words may evoke activation.20 Activation in the Heschl gyrus or the primary auditory cortex in the posterior superior temporal lobe can help confirm localization of the Wernicke area, which is located just posterior to the Heschl gyrus (Fig. 10.6). Tongue motor is often performed along with the language paradigms because injury to the inferior lateral motor gyrus can also result in speech arrest.

Language Mapping
The primary roles for preoperative fMRI are for lateralizing language dominance and localizing essential language areas. The Broca and Wernicke areas are considered essential for speech production and speech comprehension, respectively. Tumors in the frontal language areas may lead to nonfluent aphasia and normal speech comprehension, and tumors in the temporal language areas to fluent aphasia and difficulty with comprehension (e.g., picture naming). Clinical information is critical to tailor the fMRI examination and select appropriate paradigms.
Studies have shown favorable comparisons between preoperative language fMRI and invasive electrocorticography as well as intracarotid sodium amobarbital (Wada) procedures.21,22,23,24,25,26,27 Intraoperative electrical stimulation is the gold standard for language mapping, but it is highly reliant on the brain exposed by the craniotomy, cognitive effects of anesthesia, number and quality of the neuropsychological paradigms, and cooperation of an awake patient. A meta-analysis of 442 patients reported by Dym et al28 reported 80% concordance between fMRI and Wada testing in 91% of the studies they reviewed. In addition, fMRI showed 88% sensitivity for detecting left language dominance and 83% sensitivity for detecting non-left language dominance. High sensitivity and specificity for language localization were observed despite the wide range of paradigms, experimental procedures, data analysis, and interpretations across studies. Other investigators have confirmed consistently good concordance between fMRI and Wada testing, mostly in epilepsy patients.24,25,29,30 The risks of Wada testing are not trivial—in a review of 677 patients, Loddenkemper et al31 reported complications in 10.9%, including encephalopathy (by far the most common at 7.2%), seizure, stroke, and transient ischemic attack. At our institution (a National Cancer Institute–designated cancer center), Wada testing has been supplanted by fMRI for language lateralization and localization. Although Wada testing allows language lateralization without localization, it remains the test of choice for memory lateralization and is considered superior for preoperative planning when memory is a primary concern, such as in patients with temporal lobe epilepsy.
Language fMRI performed in brain tumor patients has been described as successful on a technical basis in 100% and successful for localization and lateralization in 98%.32 Language hemisphere dominance is typically described by calculating the laterality index from the number of voxels in the left hemisphere (nV×L) and right hemisphere (nV×L), where LI = (nV×L – nV×R)/(nV×L + nV×R). This estimates the relative contributions to language activation from the left and right hemispheres and is therefore affected by the statistical threshold used to create the activation maps, although threshold-independent approaches have recently been advocated.33 Evaluation of regional as well as hemispheric laterality indices may be useful to better elucidate interindividual spatial heterogeneity in language lateralization.30,34 This is especially relevant for brain tumor patients, where large local changes in BOLD signal may result from tumor-related neurovascular decoupling, hemorrhage, and other sources of susceptibility artifact.35
In clinical practice, most patients show clear left language dominance with left hemisphere Broca area and Wernicke area confirmed across multiple paradigms. A right hemisphere Broca area analogue is often identified in strongly left hemisphere language dominant patients. This nondominant activation is associated with visual spatial processing and prosody functions related to the rhythm, tempo, emotion, pitch, and intonation of speech. Prosody deficits or aprosodia may present a severe handicap for some patients. True right hemisphere dominance manifest with the right Broca area and the right Wernicke area is unusual, even in left-handed individuals. The phonological processing recruited by word-generation paradigms may yield lateralization results that are superior to those elicited by semantic fluency, word-reading, and picture-naming paradigms.
Performance of multiple paradigms is recommended to improve the success and reproducibility of fMRI. Language paradigms may be biased toward the frontal or the temporal language areas; however, they usually elicit activation in both areas (Fig. 10.4). Benke et al36 described superior agreement for frontal laterality indices, rather than temporal laterality indices between fMRI and Wada testing. Other groups have described higher laterality indices for the Broca area using word-generation and rhyming paradigms instead of sentence-completion or sentence-listening comprehension paradigms,33,37,38 and suggested that the expressive paradigms are sufficient for language lateralization and localization. Determining language lateralization in the receptive language areas with fMRI remains more problematic,33 probably due to the wider distribution of language comprehension functions through the brain, as well as suboptimal receptive and semantic paradigm designs. The combined paradigm analysis is useful in identifying language areas involved in general language functions19,26,27,39 and has been advocated as superior for language localization, rather than using a single paradigm, which may only identify language areas specific to the particular paradigm performed.26
Activation of secondary or supportive portions of the complex language neural network is nearly always present during fMRI. The locations, functions, and sequelae of damage to important secondary language areas40,41,42,43,44,45 are summarized in Table 10.2. Similar to the primary areas, these secondary areas become relevant when located in close proximity to a tumor. For example, localization of the SMA when planning resection of a tumor in the superior frontal gyrus will help facilitate patient counseling. The SMA proper is responsible for planning motor movements and the pre-SMA for linguistic planning; injury to the SMA may cause SMA syndrome with profound but transient speech deficits or mutism. The middle frontal gyrus has a prominent role in phonological fluency and verbal working memory, in conjunction with the Broca area, premotor area, and SMA.
Area | Brodmann area | Location | Function | Sample deficits |
Middle frontal gyrus (dorsolateral prefrontal cortex, premotor area) | 46 | Superior to Broca area | Verbal working memory | Dysarthria, anomia |
Supplementary motor area (SMA) | 6 | Superior frontal gyrus, with language SMA anterior to motor SMA | Complex coordinated movements, retrieval of motor memory | Transient speech paucity, mutism |
Insula | 13 | Deep to sylvian fissure | Phonological and semantic processing, overt speech paradigms | Word finding difficulty, speech apraxia |
Angular gyrus and supramarginal gyrus | 39 and 40 | Wrapped around posterior portion of sylvian fissure | Semantic processing, attention to phonological relations, working memory | Alexia, agraphia, semantic aphasia |
10.2.3 Challenges in fMRI
There are many potential challenges in fMRI that require attention to proper patient preparation, protocol optimization, and data processing standardization.
Patient Preparation
fMRI requires active patient effort to yield meaningful results. This contrasts most MRI sequences where patients are only required to remain motionless (or cooperate with breathing instructions for nonneurologic protocols), and is superimposed upon the usual MRI constraints such as claustrophobia. Patients with brain tumors are often impaired, especially those who present with tumors located near eloquent brain areas. Paradigms must be selected for individual patients based on their clinical signs and symptoms, and location of the tumor on anatomical images. Results are often improved when the paradigms are rehearsed with the patient before entering the MRI suite. Monitoring BOLD signal on the scanner in real-time during paradigm delivery helps confirm that the patient is performing the specified paradigm.
Technical Considerations
fMRI requires balance between image signal and noise, and spatial and temporal resolution. Rapid imaging is necessary to capture the hemodynamic response, and T2* sensitive imaging to capture the 1 to 5% changes in T2* signal. Signal is affected by voxel volume, which is in turn affected by spatial resolution, which may be increased by decreasing the field of view and/or increasing the acquisition matrix size. Signal may also be increased by scanning at high field strengths of 3 T or more, permitting better resolution and better accuracy.46 The increased field strength allows increased polarization of proton spins, and increased magnitude of BOLD changes. Most clinical studies include whole-brain scanning and are performed at spatial resolutions less than attained on routine anatomical images. This is acceptable because the activated voxels need only be mapped at the gyrus level and are routinely coregistered onto high-resolution anatomical images. The temporal resolution of fMRI is constrained by the evolution of the hemodynamic response caused by neuronal discharges, production, and diffusion of vascular agents to cause vasodilatation; fMRI therefore has less temporal resolution than electroencephalography (EEG) and magnetoencephalography (MEG) that directly measure electrical activity caused by neuronal discharges on the order of milliseconds rather than seconds.47
Neurovascular Decoupling
fMRI is dependent on neurovascular coupling, the relationship between neuronal activation, and changes in CBF, with the changes occurring primarily in the veins immediately removed from the local neuronal activity.48 When neurons increase their firing rate, they release neurotransmitters that are removed by astrocytes. Vasodilators released by the astrocytes lead to increases in blood flow, and therefore increased concentration of oxygenated hemoglobin in the region of increased firing.
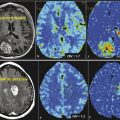
Intracranial pathologies may impede the normal hemodynamic response, such that an increase in neuronal firing will not cause a subsequent increase in blood flow or oxygenated hemoglobin. This phenomenon has been described in patients with arteriovenous malformations, high-grade gliomas, and high-grade vascular stenoses.49,50,51 The marked tumor angiogenesis and neovascularity in high-grade gliomas such as glioblastoma may already be maximally dilated and unable to respond to the increased neuronal activity with any further dilatation. The functional activity may be undetectable due to physiological and/or technical noise despite optimal patient cooperation and technique. In these situations, where negative results are unreliable (i.e., is the expected function truly absent vs. just not measureable), evaluation of the normal contralateral side may be helpful (as for inferring the location of the motor cortex) or of other areas of activation (e.g., activation in the left Wernicke area should suggest left language dominance even if a frontal lobe tumor obscures the Broca area). The BOLD signal may also be affected by pharmacological agents—increased by caffeine and decreased by antihistamines.52
fMRI Postprocessing and Interpretation
fMRI postprocessing involves (1) image preprocessing to remove motion artifacts, align slices, and coregistration to anatomical images; and (2) statistical analysis of the changes in BOLD signal. Although the postprocessing efficacy may vary according to the manufacturer and vendor software, activation maps have been described as overlapping or partially overlapping in 100% of motor paradigms and 87% of language paradigms.53 This suggests that reliable results may be obtained in the routine clinical setting with standard tools in the vast majority of cases, although advanced research tools such as Analysis of Functional NeuroImages (AFNI, http://afni.nimh.nih.gov.easyaccess1.lib.cuhk.edu.hk/), Statistical Parametric Mapping (SPM, http://www.fil.ion.ucl.ac.uk/spm/), and FMRI of the Brain Software Library (FSL, http://fsl.fmrib.ox.ac.uk/fsl/) are often better for complex cases such as when severe motion artifact is present, when complex paradigm designs with unequal active/rest states are used, or when an independent component analysis is more useful than the standard t-test, general linear model, or cross-correlation methods.54
Statistical analysis relies on detection of local alterations in the BOLD signal when performing the paradigm as compared to baseline. The redundancy of the block paradigm design helps improve signal-to-noise to identify these small changes in BOLD signal. Unfortunately, since results are generated using a within-patient analysis each time, selection of appropriate p-values and display of the results vary according to patient and scan, limiting between-patient comparisons and group analysis. And despite the acceptance of certain eloquent areas as essential, such as the primary motor cortex, even relatively simple motor paradigms usually produce activation in other areas of the brain involved in motor planning and coordination such as the SMA, premotor area, and cerebellum. This is a common issue inherent in fMRI, which will often reveal the areas supportive of the function being tested—and potentially confound localization of the essential areas.47 Rather than identifying areas involved in generating a specific function, direct electrical stimulation reversibly interrupts function to predict the deficit that would result if that specific area of the brain were resected. Determining relevant thresholds and p-values and interpreting the functional maps thereby require an experienced operator to yield meaningful results.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree