10 Mesenteric Ischemia
Guy E. Johnson
10.1 Introduction
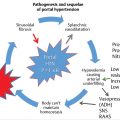
Mesenteric ischemia is an unusual clinical entity with several causes. The etiology of acute mesenteric ischemia (AMI) generally falls into one of several categories: acute arterial occlusion (embolic or thrombotic), portomesenteric venous thrombosis (PMVT), or nonocclusive mesenteric ischemia (NOMI). Chronic mesenteric ischemia (CMI) is a different condition with a separate presentation. Less common causes of AMI include vasculitis and collagen vascular diseases, trauma with vascular injury, and mechanical bowel obstruction with torsion of the vasculature. 1
Because patient presentation, prognosis, and treatment options differ for the various subtypes of mesenteric ischemia, it is important to differentiate among these distinct causes. This chapter will focus on the most common categories of mesenteric ischemia.
10.2 Epidemiology and Significance of AMI
AMI is a relatively rare but often devastating clinical entity. AMI is a rare cause for hospital admission but may be seen in as many as 30% of patients undergoing abbreviated (damage control) laparotomy for acute abdomen. 2 The mortality of AMI varies depending on the cause of the condition but can reach 70% or higher despite aggressive treatment. 3 In all cases, the prognosis is generally poor. The high mortality associated with AMI has not changed significantly over the last several decades. 3 The difficulty in establishing a diagnosis of AMI may contribute to its poor prognosis, as delays in diagnosis and treatment are associated with increased mortality. 4
10.3 Pathophysiology of Acute Mesenteric Ischemia
The splanchnic circulation receives approximately 25 to 35% of the cardiac output, depending on metabolic demands. 5 Whatever the cause, ischemic compromise of splanchnic blood flow results in a mismatch between the bowel metabolic oxygen requirement and oxygen supply. Initial ischemic changes in the bowel are confined to the metabolically more active superficial mucosa, which receives most of the blood flow to the gut. 1 , 6 The ischemic mucosa becomes more permeable, allowing passage of macromolecules into the bowel wall and lumen and resulting in fluid distention. This may progress to sloughing of epithelial cells from the tips of the villi. 6 Computed tomography (CT) findings at this stage may include a dilated lumen with wall thickening from edema (▶ Fig. 10.1); hemorrhage and ulcerations may also be present. The amount of bowel wall attenuation seen on imaging is dependent on whether edema (low density) or hemorrhage (high density) predominates. Bowel wall enhancement is also variable and may not reflect adequate perfusion. 1 , 7 As ischemia continues, changes progress to the bases of the villi and lead to mucosal necrosis; however, if ischemia is halted, this mucosal necrosis can heal completely. 7 Without correction, the process continues and the deeper layers of the bowel wall become involved. Involvement of the submucosa and muscularis propria ultimately results in transmural bowel infarction and its very high associated mortality rate.

10.4 Imaging Diagnosis of Acute Bowel Ischemia
The use of invasive catheter arteriography to establish the diagnosis of occlusive mesenteric ischemia has largely been replaced by CT evaluation, which is noninvasive and more widely available. 8 In addition to its expedience, CT has the added potential benefit of suggesting an alternative diagnosis in patients with abdominal pain. 9
Ideally, CT evaluation in mesenteric ischemia is biphasic, with both arterial and venous phases. Multidetector CT allows for multiplanar reformatted images to view mesenteric vessels in multiple planes. This may increase the ability to identify abnormalities such as an embolus lodged within the trunk of the superior mesenteric artery (SMA). CT findings suggestive of mesenteric ischemia are shown in ▶ Table 10.1. Some signs such as bowel wall thickening, luminal dilation, and abnormal enhancement of intestinal mucosa have a lower specificity, whereas other signs such as pneumatosis intestinalis are less ambiguous. However, even though pneumatosis intestinalis and portomesenteric venous gas (▶ Fig. 10.2) are commonly cited as specific and ominous CT signs of mesenteric ischemia, these findings are not absolutely specific for mesenteric ischemia and do not necessarily confer a poor prognosis. 7 Decreased or absent bowel wall enhancement is an ominous sign that can be seen in cases of embolism and mesenteric thrombosis. A detectable occlusion of the mesenteric vasculature is an exquisitely specific sign but has a poor sensitivity. Free intraperitoneal air represents a late sign of perforation from infarcted bowel. 10 In isolation, each finding may be of limited value, but when multiple signs are considered together, the sensitivity and specificity of CT angiography for detecting mesenteric ischemia are 96 and 94%, respectively. 11 , 12
Bowel wall thickening (> 3 mm) with: |
• Absent mural enhancement |
• Mural hyperenhancement |
• Mucosal hyperenhancement with submucosal edema (target sign) |
• Venous thrombosis |
• Solid organ infarction |
Superior mesenteric artery occlusion |
Mesenteric artery embolism |
Celiac artery and inferior mesenteric artery occlusion with distal superior mesenteric artery stenosis |
Mesenteric venous cutoff from torsion |
Late findings of mesenteric ischemia |
• Pneumatosis intestinalis |
• Portomesenteric venous gas |
• Pneumoperitoneum or retroperitoneal gas |
• Bowel wall thinning with absent mural enhancement |
Ancillary nonspecific findings of mesenteric ischemia |
• Mesenteric fat edema (stranding) |
• Ascites |
• Portomesenteric venous thrombosis |
• Stratification of bowel wall without mural thickening (target sign) |
• Dilated bowel |
• Focal transition from bowel dilation to collapsed bowel distally |
Source: Adapted with permission from Sandstrom et al. 1 |

Magnetic resonance angiography is quite sensitive and specific for detecting stenosis or occlusion of the SMA origin. Likewise, magnetic resonance angiography can be useful in diagnosing mesenteric venous thrombosis; however, its role is limited by availability and the time required for the examination. Ultrasound can be a useful initial screening tool to identify proximal stenoses of the SMA and celiac arteries, with threshold peak systolic velocities of 200 cm/s for the celiac and 275 cm/s for the SMA, suggesting significant stenoses. 13 The inferior mesenteric artery (IMA) is generally very difficult to identify on ultrasound, however. Doppler ultrasound has good sensitivity and specificity for detecting central mesenteric vascular occlusions, but the accuracy and feasibility of this imaging modality may be limited by the presence of bowel gas. For this reason, Doppler ultrasound is not recommended for the initial evaluation of patients suspected of having AMI. 8
Imaging signs of embolic occlusion, thrombosis, venous occlusion, and nonocclusive pathologies will be discussed below.
10.4.1 AMI Caused by Arterial Occlusion
Acute SMA occlusion accounts for 67% of all cases of AMI, 14 and acute embolic occlusion is the most common cause of AMI, representing approximately 50% of cases. 5 Most of the remaining cases are caused by mesenteric thrombosis.
Patient Presentation and Diagnosis
Early diagnosis of this condition is essential because it may decrease mortality in patients with AMI. 5 , 15 A prompt diagnosis itself represents a major challenge in AMI (of any cause) not only because of the rarity of the condition but also because of the relatively nonspecific nature of its presentation. Because the diagnosis is obscure, it is often not considered on initial presentation. 16
In patients with AMI caused by arterial embolic occlusion, rapid onset of periumbilical abdominal pain is common. The pain may be described as cramplike and continuous, is generally moderate to severe, and may even be resistant to opiates in some patients. In 30 to 50% of patients, the onset of abdominal pain is sudden; in another one-third of patients, the pain begins acutely over less than 1 hour. 16 , 17 With the severity of the abdominal pain, evidence of peritonitis might be expected on physical examination; however, this is generally not an early finding (hence the classic term pain out of proportion to the physical examination). 18 Following the onset of abdominal pain in many patients, a rapid evacuation of the bowel comes in the form of diarrhea (42%), which may be bloody, as well as vomiting (71%). 17 The abdominal pain and gastrointestinal symptoms usually occur as components of a triad of symptoms in these patients: (1) pain out of proportion to the physical examination, (2) vomiting and diarrhea, and (3) an obvious source of an embolus such as atrial fibrillation or recent myocardial infarction. 17 Given the overlap with cardiovascular disease, patients with mesenteric ischemia generally present in their sixth to eighth decade of life.
Because atherosclerosis is generally the cause in patients with AMI caused by arterial thrombosis, the thrombosis generally occurs with longstanding and progressive narrowing of the artery. Therefore, immediate symptoms may occur in the setting of insidious symptoms of CMI (discussed below).
Laboratory abnormalities are nearly always present in patients with this condition. Patients may demonstrate evidence of metabolic acidosis, with high lactate levels and leukocytosis. 19 Assessment of the D-dimer level may be useful, as a normal value may exclude the diagnosis of mesenteric ischemia. 20 Unfortunately, however, there exists no rapid laboratory test that is pathognomonic or sufficient to establish the diagnosis. 12
In cases of embolic occlusion, the thromboembolus lodges several centimeters past the SMA origin as the vessel narrows distal to the takeoff of the first jejunal branches and middle colic artery. The SMA may be more prone to embolic occlusion because of its relatively acute takeoff from the aorta. CT images in these patients may demonstrate a discrete filling defect (thromboembolus) within the SMA (▶ Fig. 10.3); this filling defect may show a convex proximal surface and can be partially or totally occlusive. Because the jejunal branches and the middle colic artery may be spared, the corresponding bowel may have a normal appearance at CT. Most emboli are cardiogenic; therefore, intracardiac thrombi may also be identified on CT when patients are evaluated for mesenteric ischemia. Synchronous emboli are evident in approximately a quarter of patients. 17

Treatment
The most common management for acute embolic mesenteric ischemia is surgery. Patients with advanced disease and infarcted bowel who present with peritonitis or shock may proceed to laparotomy even if the diagnosis is not fully established preoperatively. Although invasive, surgical revascularization affords the benefit of direct visual inspection of the bowel to assess for viability. The bowel is evaluated at laparotomy; if the bowel is deemed nonviable, it is resected. In addition to visual inspection and clinical judgment, several other techniques are used for the assessment of viability, including palpation or Doppler ultrasound of distal arterial arcades and injection of fluorescein. Unfortunately, the sensitivity of such clinical assessment is relatively poor, and “second-look” laparotomy may be necessary to resect more nonviable bowel. 21 Revascularization with a bypass graft can be performed via an antegrade (using the supraceliac aorta as inflow) or retrograde (using the common iliac artery as inflow) approach. Balloon embolectomy is an alternative to bypass that can be performed with or without patch angioplasty of the superior mesenteric arteriotomy. 22 Retrograde stenting after open exposure of the SMA has also been used. 23 Supportive measures may include fluid resuscitation to counteract fluid shifts from third spacing and initiation of broad-spectrum antibiotics to combat bacterial translocation across the ischemic intestinal wall. Anticoagulation with intravenous unfractionated heparin helps to prevent further clot propagation; this medication can be titrated off quickly if surgery becomes necessary.
Endovascular techniques for mesenteric revascularization in acute embolic mesenteric ischemia have gained interest in recent years because of reports of relatively favorable outcomes. 23 , 24 , 25 Catheter-directed thrombolysis, aspiration embolectomy, and angioplasty have all been used with good technical success. Vasodilators may be of benefit for cases in which poor flow is compounded by arterial vasospasm (▶ Fig. 10.3d). However, the literature describing the endovascular treatment of acute occlusive mesenteric ischemia remains limited. Relatively few comparative studies have been performed, and none of these studies were randomized. 24 , 25 , 26 , 27 Nonrandomized comparisons between endovascular and open approaches have demonstrated lower rates of complications such as respiratory failure (27 vs. 64%) and renal failure (27 vs. 50%) with endovascular techniques. 27 In one study, mortality was significantly lower (36 vs. 50%) in the endovascular cohort if patients were able to avoid surgical revascularization compared to the group initially treated with open surgical revascularization. There is also some evidence that if bowel resection is required, endovascular treatment before resection may decrease the length of bowel resected. 27 However, these results must be interpreted with caution given the quality of the data.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree