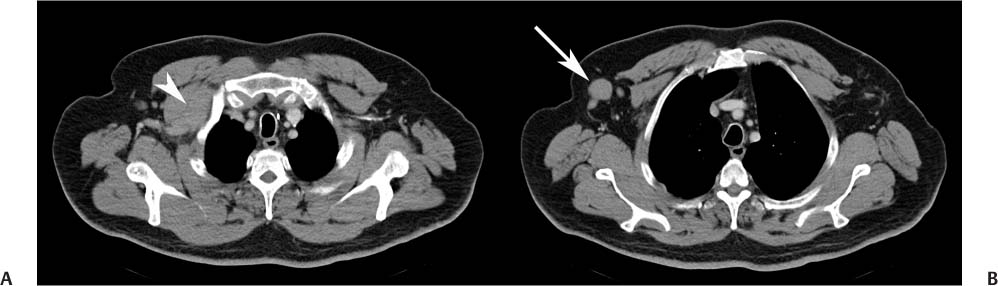
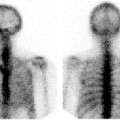
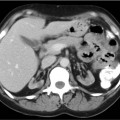
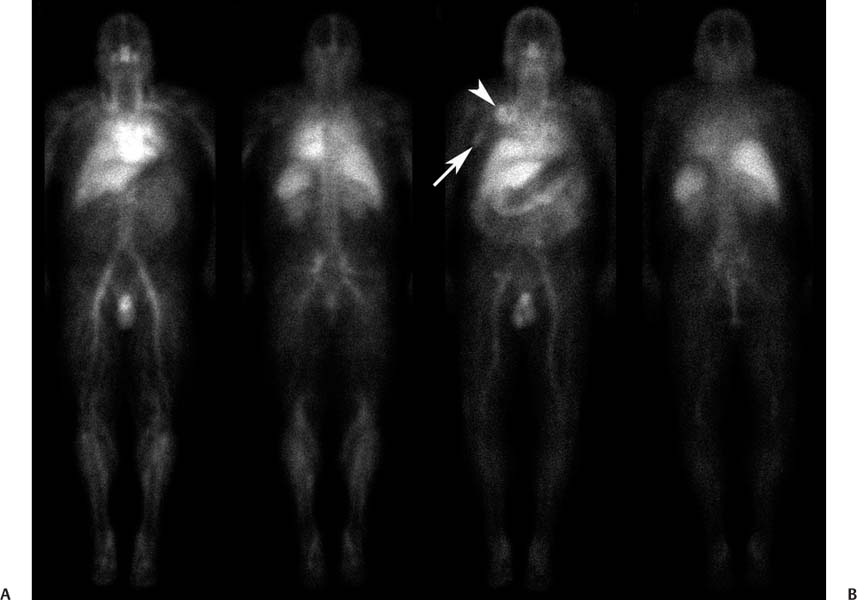CASE 101 A 30-year-old man with refractory low-grade non-Hodgkin lymphoma is referred to nuclear medicine for radioimmunotherapy. Fig. 101.1 Fig. 101.2 • 111In-ibritumomab tiuxetan 5 mCi intravenously. Planar imaging with a medium-energy collimator at 2 hours (Fig. 101.1A) and 48 hours (Fig. 101.1B). The patient was administered unlabeled rituximab, 250 mg/m2, to bind peripheral binding sites. He was then administered 111In-ibritumomab tiuxetan, 5 mCi, and imaging was performed at 2 hours (Fig. 101.1A, anterior and posterior images) and 48 hours (Fig. 101.1B anterior and posterior images) to confirm appropriate biodistribution. This was indeed demonstrated, with uptake predominantly in the blood pool, liver, and spleen on the early images, and less blood pool activity (e.g., see the heart) and persistent liver and spleen uptake on the delayed images. In addition, the delayed images reveal uptake in two sites of disease, (A) a right supraclavicular lymph node (arrowhead) and (B) a right axillary lymph node (arrow), also shown on the CT scan (Fig. 101.2). Seven days following the administration of the 111In-ibritumomab tiuxetan, the patient was again administered unlabeled rituximab, 250 mg/m2, to bind peripheral binding sites. This was followed by the administration of a therapy dose of ibritumomab tiuxetan labeled with the β– emitter 90Y-ibritumomab, 0.4 mCi/kg, by slow intravenous injection over 10 minutes. In the past few years, therapies have been developed that use monoclonal antibodies as vehicles to deliver radioisotopes to surface receptors on tumor cells, a process known as radioimmumotherapy. The premise is that radioactivity can be targeted to disseminated tumor sites while radiation to nontarget organs is minimized. Numerous radiolabeled antibodies have been developed for both hematologic and solid tumors. The most successful have been two agents directed at the CD20 antigen of B-cell lymphomas, 90Y-ibritumomab tiuxetan (Zevalin, Spectrum Pharmaceuticals) and 131I-tositumomab (Bexxar, GlaxoSmithKline). Both are registered for the treatment of relapsed or refractory low-grade, follicular, or transformed non-Hodgkin lymphoma, including rituximab-refractory disease.
Clinical Presentation
Technique
Image Interpretation
Therapy
Discussion
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree