11 Benign Biliary Strictures
Baljendra S. Kapoor and Eunice Moon
11.1 Introduction
Benign biliary strictures can be caused by multiple conditions. Most commonly, benign biliary strictures are associated with surgery, resulting from biliary injury during cholecystectomy, from fibrosis after biliary–enteric anastomosis, or from complications after liver transplant. Benign strictures can also result from chronic pancreatitis, Mirizzi′s syndrome, primary and secondary sclerosing cholangitis, autoimmune immunoglobulin G4 (IgG4) cholangitis, and chemotherapy-induced sclerosing cholangitis. Infectious causes of biliary strictures include acquired immunodeficiency syndrome (AIDS)-related cholangiopathy and oriental cholangiohepatitis (pyogenic cholangitis). 1
11.2 Clinical Presentation
Clinically, benign biliary strictures can be completely asymptomatic. Symptomatic biliary strictures present with clinical and biochemical evidence of biliary obstruction. Typically, the symptoms are related to obstructive jaundice and can include icterus, clay-colored stools, itching, and bradycardia. Biochemical changes include elevation of liver enzyme levels, including total and direct bilirubin and alkaline phosphatase levels. Chronic low-grade biliary obstruction can lead to recurrent cholangitis, stone formation, and even cirrhosis and end-stage liver disease.
11.3 Imaging
The role of imaging in the patient presenting with symptoms of biliary obstruction is to characterize and detect the level of obstruction and to determine the nature of the obstruction. On imaging, benign strictures appear to be smoother, with more symmetric borders and tapered margins, and tend to be shorter than malignant strictures. 2 Malignant strictures of the common bile duct (CBD), on the other hand, demonstrate asymmetry and irregularity with shouldered margins. 2 , 3
Ultrasound (US) is usually the first imaging modality used to screen patients presenting with obstructive jaundice. Dilation of the biliary system proximal to the stricture can be identified on US, as well as other pathologies such as biliary stones, intrahepatic abscesses, intra- and extrahepatic masses such as pancreatic pseudocyst, pancreatic mass, and lymph node mass. In patients who have undergone liver transplant, routine duplex US of the hepatic artery may reveal hepatic artery thrombosis or stenosis. US is limited in its ability to identify the underlying cause of the obstructive jaundice and is highly operator dependent.
Contrast-enhanced computed tomography (CT), on the other hand, is not operator dependent and can identify biliary dilatation, the level of obstruction, and often the underlying etiology of the obstructive jaundice such as CBD stone, acute pancreatitis, chronic pancreatitis, and pancreatic pseudocyst. Contrast-enhanced CT can also identify malignant changes associated with the obstruction, including wall enhancement, greater degree of proximal dilatation, wall thickness of greater than 1.5 mm, total length of stricture, 2 hilar lymphadenopathy, ascites, and distant metastatic lesions. In addition, CT angiogram can evaluate the patency of the hepatic artery and portal vein in post–liver transplant patients, and aid in diagnosis of post-transplant biliary stricture.
Magnetic resonance imaging (MRI) and magnetic resonance cholangiopancreatography (MRCP) have the distinct advantage of being able to evaluate the biliary system and the biliary enteric anastomosis in the immediate postoperative period. MRCP is highly sensitive for the diagnosis of biliary obstruction and is the imaging modality of choice for identifying the level of obstruction; this modality can also delineate recurrence of the primary pathology (e.g., primary sclerosing cholangitis). However, MRCP has a large range of sensitivity for differentiating between benign and malignant strictures (30–98%). 4 , 5 , 6 Furthermore, contrast-enhanced MR angiography (MRA) may be useful in detecting hepatic artery thrombosis or stenosis in patients who have undergone liver transplant. Similar to CT, MRI can detect fluid collections, ascites, and portal vein thrombosis.
Although less commonly used for the diagnosis of malignant biliary strictures, positron emission tomography (PET) with 18F-FDG (fludeoxyglucose) has been shown to be highly sensitive and specific for differentiating between benign and malignant strictures. 7
Endoscopic retrograde cholangiopancreatography (ERCP) has 90 to 95% sensitivity in detecting biliary complications after liver transplant. 8 These sensitivities are similar to those seen with MRCP, but ERCP is more invasive. ERCP has limited use in patients who have undergone biliary enteric anastomosis, and offers limited visualization of the duct distal to the level of pathology. On the other hand, ERCP is potentially therapeutic as well as diagnostic, allowing decompression of the obstruction with possible balloon dilation and placement of endoscopic stent. 9
With highly improved cross-sectional imaging techniques, percutaneous transhepatic cholangiography (PTHC) is rarely used only for diagnostic purpose. PTHC is typically performed in combination with percutaneous transhepatic biliary drainage (PTBD) based on the clinical and cross-sectional imaging findings. PTHC/PTBD is usually performed in cases that are unsuitable for endoscopic approach or in which the endoscopic approach has failed. Most interventional radiologists require a platelet count of greater than 50 and an INR (international normalized ratio) of less than 1.4 to 1.5 before performing PTHC. A right mid-axillary approach is the most commonly used approach 10 (▶ Fig. 11.1a). In the right mid-axillary approach, a 20- to 22-gauge needle is advanced in the liver, usually through the right 11th or 10th intercostal space, and directed medially and superiorly. After the stylet is removed, diluted contrast is injected, while the needle is slowly retracted to opacify a bile duct. This step is repeated in different planes, if needed. Entry into the peripheral duct is important to decrease the risk of major vascular complications. However, if the point of duct entry is not satisfactory, initial access may be utilized to opacify the biliary system and a more peripheral access may be obtained by targeting one of the peripheral ducts under fluoroscopic guidance. Imaging is performed in multiple projections to more clearly delineate the biliary stricture and biliary anatomy.

In the event of difficult right mid-axillary approach, a left-sided subxiphoid approach may be used (▶ Fig. 11.1b). Some interventional radiologists prefer the left-sided approach as their initial choice in the appropriate clinical setting. In the left-sided approach, most interventional radiologists prefer to gain initial access under US guidance. PTHC is an invasive procedure and complications include acute hemorrhage, pneumothorax, hemothorax, and/or injury to the hepatic flexure of the colon. Careful review of prior imaging studies and, if available, additional intraprocedural imaging tools can be very useful, particularly in patients with complex biliary anatomy. In addition, three-dimensional (3D) intraprocedural imaging tools such as CBCT (cone-beam computed tomography) may be used to further assess the needle path in difficult or complex cases before placing a large bore sheath or catheter.
PTHC is particularly useful in patients with leak associated with stricture where ductal decompression limits the diagnostic effectiveness of cross-sectional imaging. PTHC, although invasive, can be diagnostic and potentially therapeutic, especially in patients with noninvasive imaging with equivocal results and patients who have undergone biliary enteric anastomoses.
11.4 Causes of Benign Strictures
11.4.1 Iatrogenic
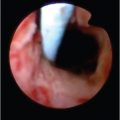
Iatrogenic injuries are by far the most common cause of benign biliary strictures. Up to 70 to 80% of all benign strictures result from iatrogenic injuries. Cholecystectomy and orthotopic liver transplant (OLT) are the most common causes of iatrogenic biliary strictures. Currently, the risk of biliary stricture after open cholecystectomy ranges from 0.1 to 0.2%; the risk after laparoscopic cholecystectomy ranges from 0.4 to 0.6%. 11 Strictures occurring after cholecystectomy usually result from accidental ligation of the common hepatic duct or CBD or from ligation of the cystic duct in close proximity to the common hepatic duct/CBD 12 (▶ Fig. 11.2a).

Strictures that occur after OLT can be anastomotic or nonanastomotic. Anastomotic strictures tend to be more focal and usually due to postoperative fibrosis or secondary to a leak (▶ Fig. 11.2b). Nonanastomostic strictures tend to be multifocal and diffuse in appearance (▶ Fig. 11.2c) and commonly caused by hepatic artery thrombosis but may also be caused by recurrence of the primary disease (e.g., primary sclerosing cholangitis, preservation injury, or infection). Ischemic strictures can involve intra- as well as extrahepatic bile ducts. 13 OLT donors can also develop biliary strictures, most commonly at the junction of the common hepatic duct and intrahepatic ducts. 14
Treatment of benign biliary strictures is determined primarily by the location of the stricture. The resectability of a benign stricture reflects the anatomic location of the stricture, and is categorized by the Bismuth classification system. 15 In general, biliary surgery can be performed easily in patients with Bismuth type I and II lesions, whereas surgical repair may be considerably more difficult in patients with Bismuth type III, IV, and V lesions.
11.4.2 Chronic Pancreatitis
Approximately 10% of benign biliary strictures are caused by chronic pancreatitis. 16 Long strictures in the distal CBD develop from recurrent pancreaticobiliary inflammation in the head of the pancreas. Furthermore, pseudocyst at the head of the pancreas can cause extrinsic biliary compression.
The clinical presentation of biliary stricture secondary to chronic pancreatitis is variable. Most strictures are asymptomatic. Symptomatic strictures present with abdominal pain, jaundice, abnormal liver enzymes, and fever with or without chills. Biliary obstruction can later progress to secondary biliary cirrhosis and choledocholithiasis.
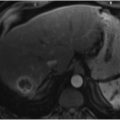
On imaging, there is smooth tapering of the distal CBD with various degrees of intra- and extrahepatic biliary dilatation (▶ Fig. 11.3). The length of the CBD stricture is reported to range from 1.6 to 5.7 cm, possibly reflecting the variable length of the intrapancreatic portion of the CBD. 17 , 18 Clinical and radiologic evidence of chronic pancreatitis (i.e., atrophy of the pancreas, calcification, a dilated main pancreatic duct, or pseudocysts) supports the diagnosis of benign strictures related to chronic pancreatitis.

Endoscopic or balloon dilatation with or without stenting is reserved for symptomatic patients with jaundice, cholangitis, or persistently abnormal liver function test results. 19 These strictures may not respond to balloon dilation. Patients with persistent or recurrent jaundice, persistent elevation of alkaline phosphatase, recurrent cholangitis, and liver abscesses may be candidates for biliary–enteric bypass surgery such as choledochoduodenostomy and Roux-en-Y choledochojejunostomy.
11.4.3 Primary Sclerosis Cholangitis
Primary sclerosing cholangitis (PSC) is a rare and chronic inflammatory disorder of unknown etiology. It is characterized by an alternating pattern of biliary stricture and mild dilatation, often described as “beaded” in appearance (▶ Fig. 11.4). PSC is most commonly seen in men in the third and fourth decades of life, often associated with inflammatory bowel disease, most commonly ulcerative colitis. Patients with PSC may remain asymptomatic; alternatively, they can present with nonspecific symptoms such as fever, loss of appetite, fatigue, and weight loss. As the disease progresses, patients present with symptoms related to cholangitis and obstructive jaundice such as itching, icterus, fever with chills, and clay-colored stool. In most patients, PSC results in end-stage liver disease and cirrhosis, necessitating liver transplant.

The MRI findings of PSC correlate with the pathologic appearance of the disease. Findings include periportal edema (high T2 signal around the portal tracts), patchy areas of peripheral arterial hyperenhancement in the early stage of the disease, and changes associated with cirrhosis in the later stage of the disease. On MRCP, PSC is characterized by multifocal intra- and extrahepatic biliary strictures with alternating areas of normal segments or minimally dilated segments. Isolated involvement of the intrahepatic biliary system is seen in approximately one-third of patients, whereas isolated extrahepatic duct involvement is rare. Prominent periportal lymph nodes may also be seen. According to Fulcher et al, 20 the sensitivity of MRCP for the detection of PSC ranges from 80 to 88% and the specificity ranges from 87 to 99%. Imaging also plays a vital role in the detection of complications such as choledocholithiasis and cholangiocarcinoma, which occurs in 3.3 to 36.4% of patients with PSC.
Medical management of PSC involves treatment with ursodeoxycholic acid and immunosuppressants, including steroids. Dominant strictures are treated with balloon dilatation. Eventually, most patients will require a liver transplant, which is associated with excellent 1- and 5-year survival rates of 90 and 85%, respectively. 21
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree