12
Interventional Radiology
Thomas A. Farrell
 Chapter Outline
Chapter Outline
Instruments and Tools of the Trade
Venous Imaging and Interventions
Hemodialysis Access Interventions
Percutaneous Biliary Drainage and Stenting
Interventional radiology (IR) is a diverse practice of patient care using minimally invasive image-guided procedures to diagnose and treat disease nonoperatively. Percutaneous diagnostic and therapeutic procedures are performed using fluoroscopy, ultrasound, computed tomography (CT), or magnetic resonance (MR) imaging for guidance. These procedures, which may be categorized as vascular (i.e., arteriography, venography) and nonvascular (e.g., drainage of abscesses, obstructed kidneys and bile ducts), are performed in an IR suite and are often done on an outpatient basis. Many procedures that were previously performed surgically are now accomplished by an interventional radiologist with less morbidity and a shorter hospital stay.
Since 1953, when Dr. Sven-Ivar Seldinger described a method of percutaneous arterial access using a hollow-core needle, guidewire, and catheter, IR has continued to evolve, as new techniques and devices are developed to enhance patient care. Technical advances have led to significant improvements in patient safety and procedural diversity. As these rapid changes in endovascular technologies continue to expand, so will the possibilities of image-guided, minimally invasive procedures.
Because IR is procedural, interventional radiologists become more involved in patient care. Many IR practices offer an active inpatient and outpatient consult service and also employ specially trained nurse practitioners and physician’s assistants as physician extenders. Patients are routinely worked up by the IR service and are subsequently followed up postprocedure. The preprocedure workup consists of patient assessment as well as evaluation of previous imaging studies (Table 12.1). Postprocedure follow-up is essential to determine whether the procedure has been successful and free of complications. This all-inclusive clinical service underlines that there is more to IR than simply doing procedures. Because procedures performed by interventional radiologists are invasive, the risk of complications is ever present. It is important that the patient be aware of these risks so that an informed consent can be made by weighing the possible risks of a procedure against its potential benefits. A physician should never place a patient in a position of risk unless the risks, benefits, and alternatives of the planned procedure have been discussed, understood, and consented to before the procedure. It is in the physician’s best interest to be honest and forthright when dealing with patients and their expectations about the outcomes of a procedure.
Table 12.1

The aim of this chapter is to explain the background, indications, and basic techniques of the procedures commonly performed in IR so that the reader will gain an understanding of how this subspecialty contributes to patient care.
INSTRUMENTS AND TOOLS OF THE TRADE
IR procedures are performed in imaging suites with fluoroscopy and digital subtraction angiography (DSA). Ultrasound, CT, and MR imaging are also utilized by the interventional radiologist.
Endoluminal and endovascular procedures require administration of a contrast agent for improved visualization. Nonionic iodinated contrast is most frequently used to delineate, radiographically, the lumen of an artery, vein, and biliary duct, gastrointestinal (GI) or urinary tract. Alternatively carbon dioxide gas or gadolinium can be used in patients with renal insufficiency or allergy to radiographic contrast agents.
There is a variety of commercially available catheters, sheaths, guidewires, angioplasty catheters, vascular stents, and caval filters and familiarity with these and their use requires training and experience. There are numerous preformed shapes and types of angiographic catheters, most of which are made from flexible plastic material such as polyethylene or polyurethane. Wire braiding may be incorporated into the catheter shaft to increase stiffness and improve its torque. Catheter diameters are measured in French (F) size, where 3F = 1 mm (outside diameter). Most angiographic catheters are in the 4F to 7F range. Aortic angiography is performed with pigtail catheters that have several side holes proximal to the tip allowing rapid flow of a contrast bolus while the pigtail loop stabilizes the catheter preventing recoil (Fig. 12.1A,B). Selective angiography (renal, celiac, and superior mesenteric arteries) is performed with a curved end-hole catheter such as a Cobra C2 (Fig. 12.1C). A variety of catheters and guidewires may be necessary during a procedure, and placement of a vascular sheath with a hemostatic valve at the site of access reduces vessel trauma and facilitates rapid catheter and guidewire exchange.
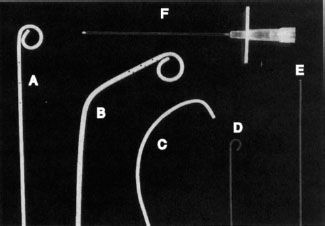
FIGURE 12.1. Tools of the trade. A: Pigtail catheter. B: Angled pigtail catheter. C: Cobra catheter. D: J-tipped guidewire. E: Straight (Bentson) guidewire. F: An 18G needle for vessel puncture.
Catheters used in the drainage of abscesses, obstructed kidneys (percutaneous nephrostomy), and bile ducts are made of polyurethane and are of greater diameter (8F to 22F) than angiographic catheters. These drainage catheters are usually placed using the Seldinger technique after which they are secured in position by deploying a locking pigtail mechanism formed by pulling on a suture that runs in the catheter shaft and is attached to its tip. The pigtail loop itself contains large side holes for drainage purposes. The smaller diameter catheters occlude more easily with debris and should be routinely changed over a guidewire every 6 to 8 weeks when continued drainage is required.
Guidewires increase the ease and safety of catheter placement. The outer shell of a guidewire consists of a very tightly wound but flexible metal spring coil. A stiff central core provides rigidity over a variable length of the guidewire. The balance between these two components dictates the handling characteristics of the guidewire. For example, the distal 15 cm of a Bentson guidewire is floppy, allowing easy coiling (Fig. 12.1E), whereas a J-tipped guidewire reduces the risk of damaging the vessel wall because of its blunt tip (Fig. 12.1D). Guidewires usually range in diameter from 18 thousandths of an inch (0.018″) to 38 thousandths of an inch (0.038″). The standard length for most wires is 145 cm, while longer guidewires (260 cm) are available to facilitate catheter exchange.
Needles used in arteriography vary in size from a 21G needle through which a 0.018-inch guidewire will pass to an 18G needle that accepts a 0.035-inch guidewire (Fig. 12.1F).
ANGIOGRAPHY
Angiography is a technique of imaging blood vessels, usually by injecting contrast material via an intraluminally placed catheter. Blood vessels may also be visualized noninvasively using computed tomography angiography (CTA) or magnetic resonance angiography (MRA), which takes advantage of the inherent contrast between flowing blood and stationary tissue.
Catheter Arteriography
Diagnostic arteriography begins by catheterizing an artery (usually common femoral or brachial) using the Seldinger technique (Fig. 12.2). After placing a hollow-core needle into the artery, a guidewire is inserted through the needle and advanced into the artery. The needle is exchanged for a vascular catheter or sheath. Subsequent catheter movement and exchange is performed over a guidewire. Sonographic and fluoroscopic guidance is often necessary using this technique. Large vessel arteriography is performed using flush catheters (pigtail). Smaller arteries are selectively cannulated using catheters of various shapes and sizes. Microcatheters are used for sub- or superselective arteriography.
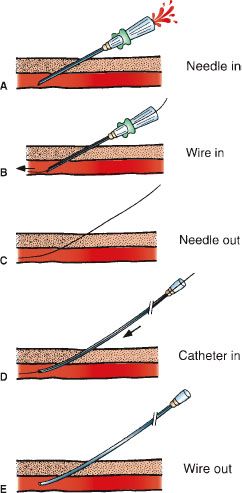
FIGURE 12.2. Seldinger technique. A: The vessel is punctured with the needle. B: A guidewire is advanced through the needle into the vessel. C: The needle is removed leaving the guidewire in place. D: A catheter is advanced over the guidewire into the vessel. E: The guidewire is removed and the catheter flushed.
After the catheter is safely positioned in the artery of choice, the guidewire is removed and contrast injected through the catheter during image acquisition usually with DSA which involves the acquisition of several mask images before injection of contrast, allowing for subsequent subtraction of nonvascular structures from the next set of images which are acquired as the contrast agent flows through the lumen of the vessel producing the arteriogram. The catheter can be exchanged or repositioned for additional imaging. After completion of the procedure, the catheter is removed from the artery and hemostasis obtained at the arteriotomy site using manual compression or a percutaneous closure device such as a nitinol clip which grasps the arterial wall externally in a purse-string fashion and closes the arteriotomy with minimal impact on the vessel diameter. Post procedure recovery time for the patient is 2 to 6 hours.
Noninvasive angiography (MRA/CTA) is gradually replacing diagnostic catheter arteriography, except where intervention is expected or other examinations are inconclusive. Pulmonary arteriography, formerly considered the gold standard in the diagnosis of pulmonary embolism (PE) has largely been replaced by CT, which has a high specificity and sensitivity (see Figure 3.50C). Both CTA and MRA are widely used in the evaluation of aortic, visceral, renal, and peripheral arterial disease (PAD) (Fig. 12.3). However, the administration of gadolinium commonly used in MRA, is associated with a higher incidence of nephrogenic systemic sclerosis in patients with renal impairment.
Peripheral Arterial Disease
Generally, the diagnosis of peripheral arterial disease (PAD) has already been made by the time an arteriogram is requested. The initial evaluation includes an assessment of the patient’s symptoms (intermittent claudication, rest pain, nonhealing ulcer), physical examination, and a review of the noninvasive imaging tests, such as CT, MR, duplex ultrasonography, and segmental limb pressures before proceeding to angiography. Rather than being an end point, the angiogram helps formulate a comprehensive plan in the patient’s subsequent management as it evaluates the extent and severity of disease and provides a road map for intervention (balloon angioplasty, stenting, surgery, etc.). Patients with diabetes may present with a more advanced stage of ischemia as they are prone to developing peripheral neuropathy that may mask the above symptoms. Diabetics also tend to have a greater prevalence of small vessel (infrageniculate) disease, which is more difficult to treat surgically and contributes to a less favorable long-term prognosis compared to other causes of PAD.
Arteriographic examination of patients with PAD may be divided into three anatomic regions: Aortoiliac, infrainguinal, and infrageniculate. Abdominal aortic aneurysms (AAA) occur most commonly below the level of the renal arteries. The number of renal arteries should also be noted, as should the presence of stenoses in these vessels. Bilateral oblique views of the pelvis should be obtained during the arteriogram, as hemodynamically significant stenoses can be missed if only a frontal view is performed.
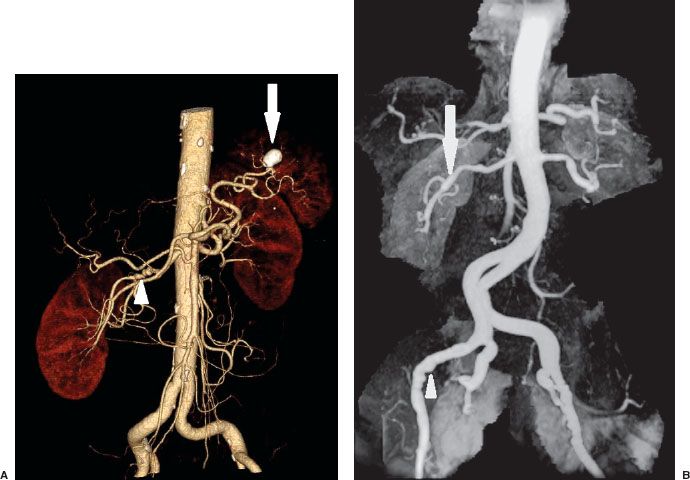
FIGURE 12.3. A: CTA of Abdomen. Volume rendered images of the abdomen show the abdominal aorta and its branches. There are multiple stenoses in the mid right renal artery consistent with fibromuscular dysplasia (arrowhead). There is also a calcified splenic artery aneurysm (arrow). B: MR angiography of the abdomen and pelvis shows multiple stenoses of both renal (arrow) and external iliac arteries (arrowhead) consistent with fibromuscular dysplasia.
In general, arterial stenoses are not regarded as significant unless they reduce the lumen diameter by 50% angiographically. Measurement of a pressure gradient across it can more accurately assess the significance of a arterial stenosis, with a 10-mm Hg gradient or greater being regarded as significant and worthy of further treatment such as angioplasty or stenting. If the gradient is less than 10 mm Hg, a vasodilator such as nitroglycerin may be given intra-arterially to simulate exercise and possibly unmask a significant stenosis. Common sites for endovascular intervention include the carotid, renal, aortoiliac, and femoropopliteal arteries.
In the absence of satisfactory femoral pulses bilaterally, either the brachial or radial arteries can be used for percutaneous access.
VASCULAR INTERVENTIONS
Thrombolysis
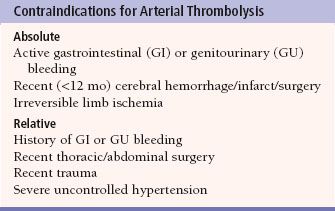
Thrombolysis is the process of dissolving blood clot in order to re-establish patency of an occluded (thrombosed) vessel, using drugs such as urokinase and tissue plasminogen activator (t-PA). These drugs are infused directly into the thrombosed grafts and vessels via catheters to ensure a very high local concentration of the drug. Contraindications for thrombolysis include internal bleeding, recent intracranial hemorrhage, or surgery (Table 12.2).
Complications of thrombolysis include bleeding and distal embolization of thrombus. The cumulative probability of major complications increases with duration of infusion, rising from less than 10% after 16 hours to more than 30% at 40 hours. Once thrombolysis is complete, angioplasty, stenting, or surgery can be used to treat any underlying vessel stenoses that contributed to the occlusion. Treatment of an acute native arterial occlusion is better done mechanically, either surgical embolectomy or catheter-directed aspiration.
Table 12.2
Balloon Angioplasty
Percutaneous transluminal balloon angioplasty (PTA) has become an established technique in the treatment of vascular stenoses due to atherosclerotic plaque and fibromuscular dysplasia. The precise pathophysiologic mechanism of PTA in atherosclerotic plaque is controversial. However, most agree that PTA results in a controlled plaque and intimal fracture with localized dissection into the underlying media thereby increasing the intraluminal diameter. The plaque, intima, and media are subsequently remodeled to give a smoother endoluminal surface. The appropriate angioplasty balloon catheter should be chosen so that its inflated diameter is the same size or slightly larger than the adjacent nondiseased vessel. Initially, the stenosis is crossed with a guidewire that is left across the lesion until the procedure is finished. Heparin and nitroglycerin may be given intra-arterially to prevent thrombosis and vessel spasm, respectively. The angioplasty balloon is advanced across the stenosis, inflated, and deflated slowly under fluoroscopic guidance. Repeat angiography and pressure measurements should be obtained to evaluate the results of angioplasty. Suboptimal angioplasty results may require placement of an endovascular stent.
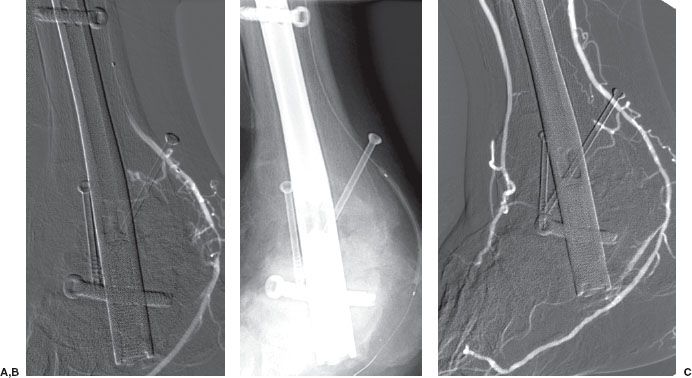
Iliac artery angioplasty improves inflow to the lower limb and requires balloons that are 7 to 10 mm in diameter. Again, a guidewire is left across the stenosis during the procedure, the success of which is judged on angiographic and hemodynamic criteria. Stent placement should be considered if the postangioplasty pressure gradient is greater than 10 mm Hg, there is residual stenosis of greater than 30%, or if a flow-limiting dissection is present (Fig. 12.4). Simultaneous PTA of both common iliac arteries, known as the kissing balloon technique, is effective in treating bilateral proximal common iliac artery stenoses.
Infrainguinal angioplasty (superficial femoral and popliteal arteries) is gaining clinical acceptance as patency outcomes for PTA and stenting rivals outcomes of surgical bypass procedures. Infrageniculate angioplasty (anterior/posterior tibial and peroneal arteries) is usually performed for limb salvage or to reduce the extent of an impending below-the-knee or forefoot amputation for ischemia. This technique requires a fine diameter guidewire (0.010″ to 0.018″) and angioplasty balloon (2 to 3 mm in diameter) because of the smaller vessel size (Fig. 12.5).
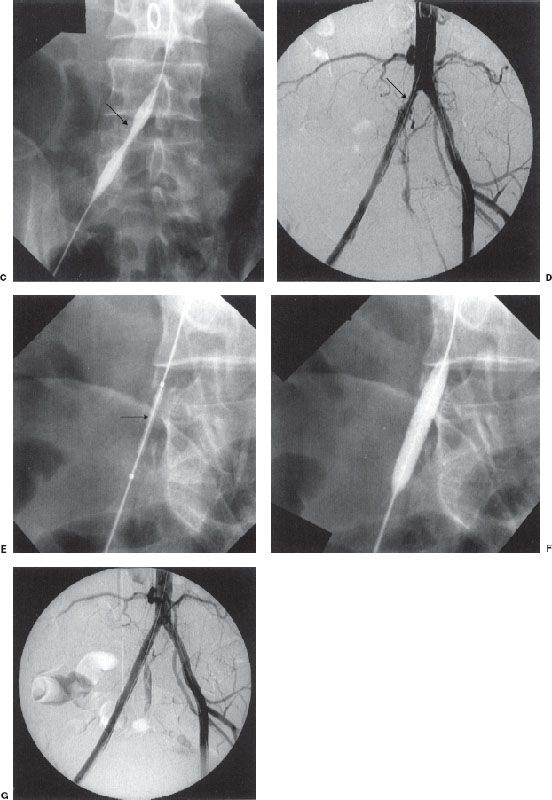
Renal artery angioplasty is usually performed with a 5- to 7-mm diameter balloon. Atheromatous disease usually involves the proximal or ostial portion of the vessel in contrast to fibromuscular dysplasia that usually affects the midportion of the vessel. The improvement in renal function and hypertension following renal artery angioplasty is equivalent to that obtained after surgical revascularization (Fig. 12.6). Renal artery stenting is performed if there is a residual stenosis or significant dissection postangioplasty (Fig. 12.7). Ostial renal artery stenoses are often stented primarily, without balloon predilatation. It has been noted that improvement in hypertension and renal function is not universal postangioplasty/stent. Cardiovascular Outcomes in Renal Atherosclerotic Lesions (CORAL) is an ongoing multicenter study funded by the National Institutes of Health, which has randomized more than 900 patients with greater than 60% stenosis to optimal medical therapy alone or optimal medical therapy plus renal artery stenting.
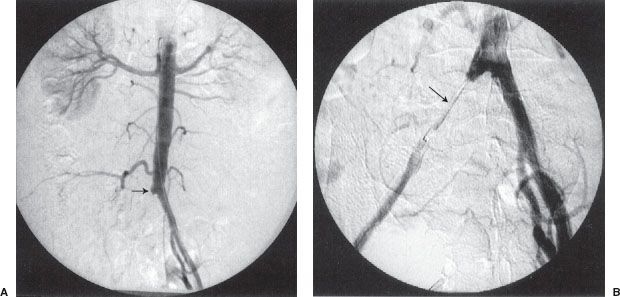
FIGURE 12.4. Arterial thrombolysis, balloon angioplasty, and stenting of a common iliac artery occlusion. A: Aortogram/pelvic angiogram shows occlusion of the right common iliac artery (arrow). B: Partial recanalization of the right common iliac artery following thrombolysis performed via an infusion catheter (arrow). C: Balloon angioplasty was performed showing residual narrowing of the balloon (arrow). D: The common iliac stenosis persisted postangioplasty (arrow). E,F: A balloon-expandable stent was deployed across the stenosis. The undeployed stent (arrow) can be seen on the distal portion of the angioplasty balloon. G: Poststenting, no residual stenosis is present.
FIGURE 12.5. Small vessel angioplasty. A: Right lower extremity angiogram, in a patient with a non healing foot ulcer post internal fixation of an ankle fracture, which shows a focal stenosis in the distal posterior tibial artery. B: The stenosis was traversed and balloon dilated with a 2-mm diameter angioplasty balloon. C: Follow up angiography showed improved flow which resulted in prompt healing of the ulcer.
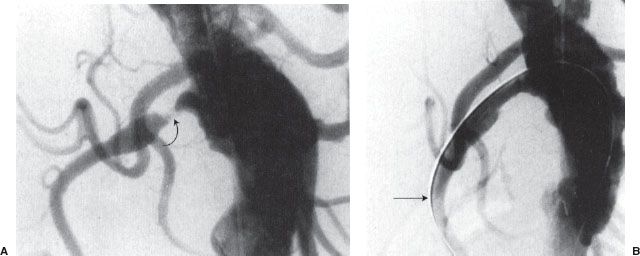
FIGURE 12.6. Renal artery angioplasty. A: Flush aortogram showing right renal artery stenosis (curved arrow). B: Residual stenosis persists postballoon angioplasty. Note that the guidewire (arrow) is left across the stenosis.
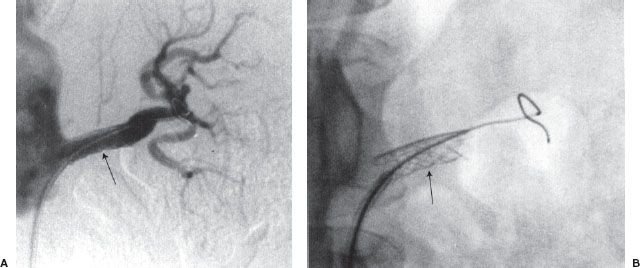
FIGURE 12.7. (A and B) Renal artery stenting. Palmaz stent (arrow) has been placed across a left renal artery stenosis.
Endovascular Stents
Peripheral Endovascular Stents
There are two main indications for endovascular stent placement: (a) A residual pressure gradient of more than 10 mm Hg postangioplasty, which is regarded as an indication for either repeat angioplasty or stent placement and (b) postangioplasty flow-limiting dissection, in which the goal of stent placement is to appose the dissected flap against the wall and improve flow. The balloon–stent combination is placed across the stenosis and the balloon is inflated, thus opening and deploying the stent. The balloon is then deflated and removed (Fig. 12.8). There are two general types of metallic endovascular stents, balloon-expandable and self-expanding. Deployment of the balloon-expandable stent is described above. Deployment of the self-expanding stent, which does not require delivery on an angioplasty balloon, involves withdrawal of a covering sheath, after which the stent expands. Postdilatation with an angioplasty balloon may be necessary. Self-expanding stents are usually more flexible than balloon-mounted stents, which is an advantage when stenting tortuous vessels (Fig. 12.9). Covered (polytetrafluoroethylene [PTFE], Dacron) stents are available for treatment of vascular injury resulting in pseudoaneurysm, hemorrhage, or arteriovenous (AV) fistula. Drug-eluting stents are increasingly being used in the treatment of superficial femoral artery stenoses. These stents are coated with drugs which prevent cellular proliferation and are designed to reduce restenosis which develops inside the stent.
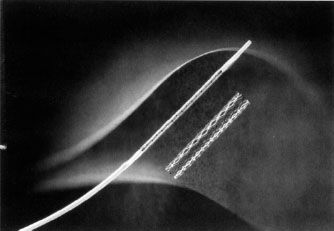
FIGURE 12.8. A balloon-expandable stent mounted on an angioplasty balloon and in its expanded form. (Courtesy of Cordis Corporation.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree