13 Generalized Bone Disease
Bone consists of three major components: the mineralized structure, the red marrow, and the yellow marrow. In the mineral component, cancellous or trabecular bone is surrounded by compact or cortical bone. Computed tomography (CT) is an excellent method to analyze the mineralized component, whereas magnetic resonance imaging (MRI) is the method of choice for the evaluation of red and yellow marrow.
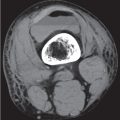
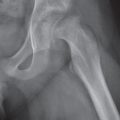
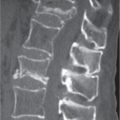
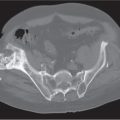
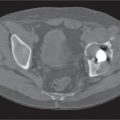
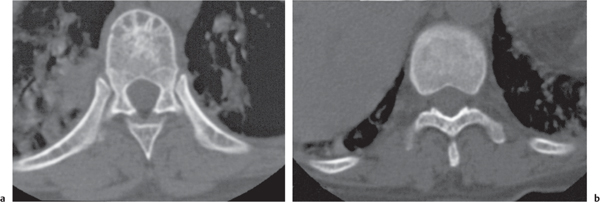
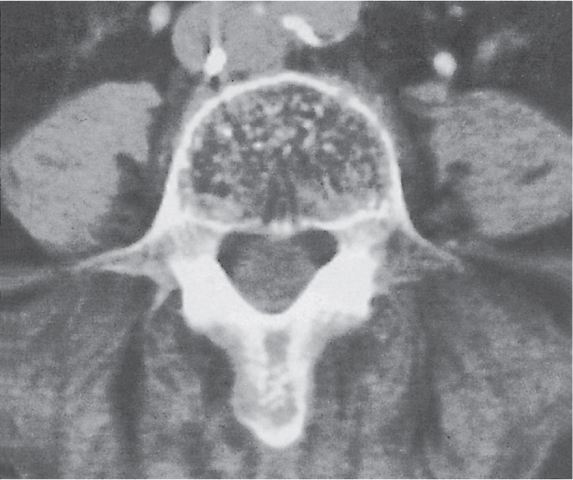
Diseases affecting either the mineralized structure or the bone marrow may increase or decrease the bony mass. Osteo-sclerosis is caused by an increased activity of osteoblasts or by osteogenic or chondrogenic tumor cells forming bonelike tissue. Diffuse osteosclerosis is most commonly associated with sickle cell disease (Fig. 13.1) and renal osteodystrophy (secondary hyperparathyroidism) (Fig. 13.2). Other causes are myelofibrosis (Fig. 13.3), fluorosis (with preferential axial skeletal involvement and ligamentous calcifications), mastocytosis, osteopetrosis, and pyknodysostosis. Diffuse osteosclerosis is common in extensive metastatic disease, especially from breast and prostatic carcinomas (Fig. 13.4), lymphoma (especially Hodgkin disease) (Fig. 13.5), and Paget disease (Fig. 13.6), but in these conditions, it is usually not uniform even with extensive involvement. Major causes of widespread or diffuse osteosclerosis in the adult are summarized in Table 13.1.
Osteopenia is defined as a decrease in bone density regardless of its etiology. The bone density correlates with both osteoid matrix mass and its calcification. Causes of generalized osteopenia include osteoporosis, osteomalacia, hyperparathyroidism, and bone marrow hyperplasia. In osteoporosis, the osteoid matrix is reduced but normally calcified. In osteomalacia, the osteoid matrix is inadequately mineralized. In hyper-parathyroidism, bone resorption is accelerated by an increased osteoclast to osteoblast ratio that is often associated with insufficient matrix calcification. In myeloproliferative disorders (e.g., thalassemia, leukemia, and multiple myeloma), the bone mass is partially replaced by the hypercellular bone marrow (Table 13.2). CT can also be used to accurately measure bone mineral content. A description of this technique is beyond the scope of this chapter, especially because this method has largely been replaced by dual energy x-ray absorptiometry (DEXA-scan).
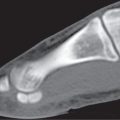
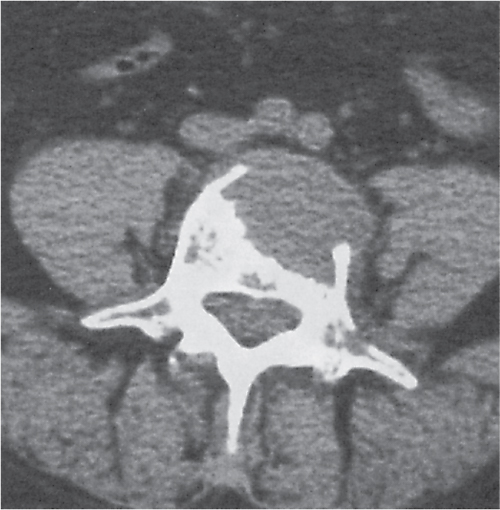
Generalized osteoporosis is characterized by cortical thinning and reduction in both the number and thickness of, preferentially, the non–weight-bearing trabeculae (Fig. 13.7). The disease is most prominent in the axial skeleton, particularly the vertebral column. The involved vertebral bodies may be wedge-shaped, biconcave (“fish vertebrae”), or compressed. Protrusion of portions of the intervertebral disk through the end plates into the vertebral body (Schmorl nodes) may also be present.
A spotty osteoporosis is occasionally found in the appendicular skeleton near a joint and indicates a more acute process. It is usually associated with disuse and immobilization or found with reflex sympathetic dystrophy (RSD, also known as complex regional pain syndrome), transient regional osteoporosis, and rheumatoid or septic arthritis. In these conditions, the osteoporosis may also present either as homogeneous juxtaarticular loss of bone density or as bandlike lucent areas in the subchondral and metaphyseal bone. Spotty and bandlike osteoporosis may at times be difficult to differentiate from malignancy, although the latter condition tends to primarily involve the cancellous bone and spare the cortical bone, with the exception of endosteal scalloping. Table 13.3 summarizes disorders with regional or localized osteoporosis.
Osteoporosis is often complicated by fractures commonly involving the lower thoracic and lumbar spine, hips, distal radius, and ribs. Insufficiency fractures may present as poorly defined irregular sclerotic bands or radiolucent lines, preferably in the pubic rami and symphysis, the supra-acetabular area, sacrum, femoral neck, and proximal and distal tibia. A cortical break is not always appreciated in these conditions. Insufficiency fractures presenting in the diametaphyses of long tubular bones as poorly defined sclerotic bands have to be differentiated from reinforcement lines (bone bars) presenting as well-defined, thin sclerotic lines with a horizontal or oblique course.
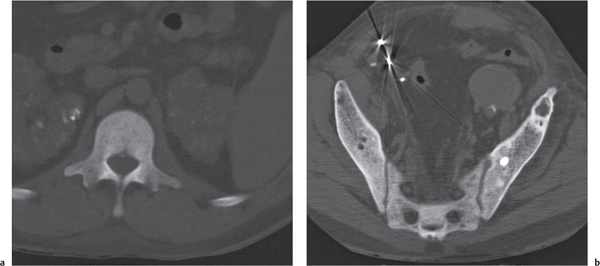
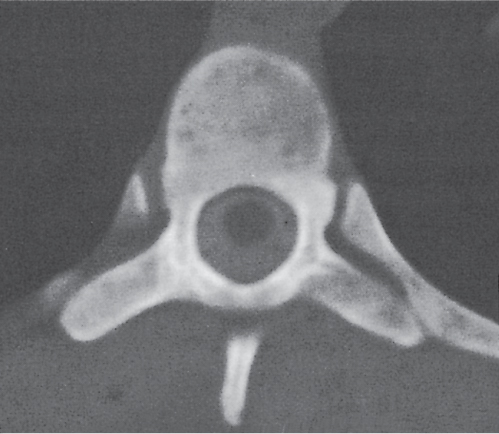
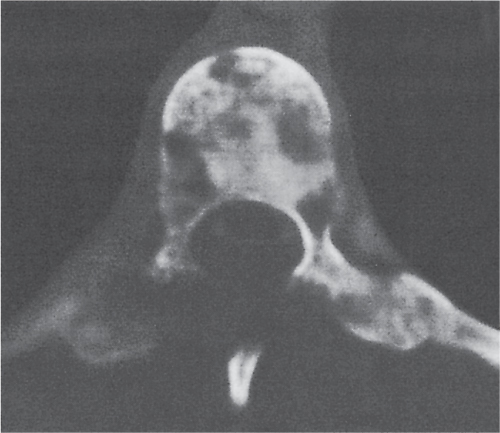
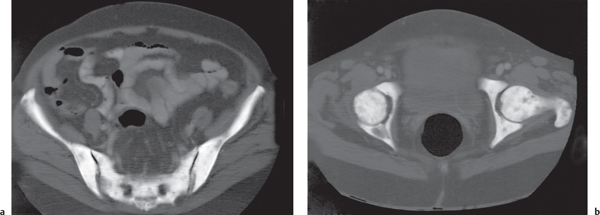
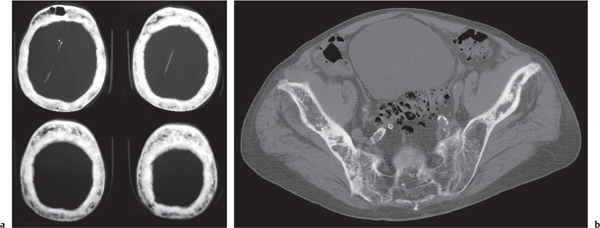
In osteomalacia, the loss of bone mass is associated with indistinct trabeculae and a fuzzy interface between cortical and medullary bone. Osseous deformities (e.g., acetabular protrusions) and pseudofractures (Looser zones) in the pubic rami, medial aspect of the femoral neck, axillary margin of the scapula, and ribs may be observed. Rickets (osteomalacia in infants and children) is diagnosed by the characteristic changes in the tubular bones that include both poorly defined and poorly calcified epiphyses, widening of the physis, and widened, cupped, and frayed metaphyses.
In hyperparathyroidism, the loss of bone density is radiographically similar to osteomalacia. Subperiosteal bone resorption (especially the radial aspect of the proximal and middle phalanges of the second and third fingers and the medial margins of the proximal tibia) is, however, virtually diagnostic. Other associated features include cortical striations (“tunneling of the cortex”), subchondral bone resorption (e.g., widening of the sacroiliac, sternoclavicular, and acromioclavicular joints and pubic symphysis), subligamentous bone resorption (trochanters, ischial and humeral tuberosities, calcanei, and distal clavicles), chondrocalcinosis, brown tumors, soft tissue calcifications (especially arterial and para-articular), nephrocalcinosis, and nephroureterolithiasis. In secondary hyperparathyroidism (renal osteodystrophy), diffuse osteosclerosis, rather than osteopenia, is usually present.
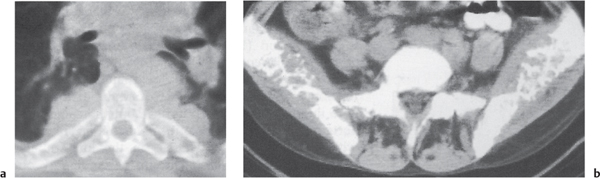
Both bone marrow hyperplasia and neoplasia may induce generalized osteopenia and/or sclerosis. In thalassemia major, a reticulated or cystic trabecular pattern with widened medullary spaces and thinned cortices is characteristic (Fig. 13.8). Other typically associated features are Erlenmeyer flask deformities (flaring of the metaphyses and epiphyses of the long bones, especially distal femora), expanded posterior aspects of the ribs, widening of the diploic space of the skull with thinning of the outer table and dense radial striations traversing the thickened calvarium (“hair on end” appearance) with sparing of the base of the occiput, and poor pneumatization of the paranasal sinuses and mastoids.
In sickle cell disease, generalized osteosclerosis may be the dominant feature, mainly involving the axial skeleton. Signs of bone infarction in the long tubular bones are commonly associated with this condition. In other anemias (e.g., iron-deficiency anemia and hereditary spherocytosis), the skeletal abnormalities caused by marrow hyperplasia are generally less severe and usually inconspicuous.
The radiologic manifestations of many lipid storage diseases (e.g., Gaucher and Niemann–Pick diseases) are similar to bone marrow hyperplasia in anemias and include a generalized osteopenia, often with coarse trabecular pattern, cortical thinning and scalloping, and Erlenmeyer flask deformities. However, in Gaucher disease, avascular necrosis of the femoral heads, calcified bone infarcts, and discrete lytic lesions with or without sclerotic margins are characteristically associated.
Leukemias are divided into acute and chronic forms. Acute forms occur in both children (peak age 2–5 y) and adults, whereas chronic forms are prevalent in patients between 35 and 60 y of age. Skeletal manifestations are most common in acute childhood leukemia and least common in chronic forms. Diffuse osteopenia may be associated with radiolucent metaphyseal bands, irregular lytic foci with or without periosteal reaction, and occasionally increased thickness of bone trabeculae and areas of osteosclerosis. Because leukemia arises from the red bone marrow, bony manifestations in adults are largely limited to the axial skeleton, whereas in children, involvement occurs frequently in both the axial and appendicular bones.
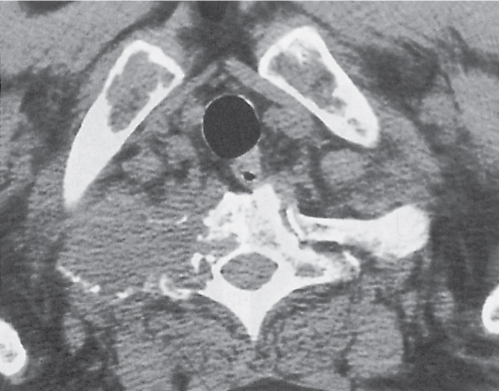
Multiple myeloma (Fig. 13.9) characteristically presents as widespread osteolytic lesions with discrete margins of rather uniform size, more frequently involving the axial skeleton. Endosteal scalloping of the cortical bone is frequently observed with involvement of the appendicular skeleton. In the spine, preferential involvement of the vertebral bodies with paraspinal extension and sparing of the posterior elements is characteristic. Diffuse skeletal osteopenia without well-defined lytic foci, however, is the most common manifestation of the disease that simulates the appearance of osteoporosis. Primary focal or diffuse sclerotic lesions are rare in multiple myeloma. They are more often the consequence of irradiation, chemotherapy, or pathologic fracture of initially lytic multiple myeloma lesions.
Diffuse osteolytic skeletal metastases (Fig. 13.10) may at times be difficult to differentiate from multiple myeloma. Lytic bony metastases tend to be more variable in size and less well defined. In the spine, both vertebral bodies and posterior elements are involved with equal frequency, whereas in the long tubular bones, endosteal scalloping is not as commonly observed as in multiple myeloma.
Diffuse osteolytic, mixed, or sclerotic lesions similar to meta-static involvement may also be encountered in both Hodgkin disease and non–Hodgkin lymphoma (Fig. 13.11). It is normally a manifestation of generalized disease, although primary bony lesions occasionally occur with non–Hodgkin lymphoma. A pproximately 20% of patients with widespread lymphoma have skeletal involvement. In Hodgkin disease, the spine, pelvis (including the hips), ribs, and sternum are most commonly affected and tend to be sclerotic, whereas in non–Hodgkin lymphoma, the lesions predominate in the appendicular skeleton and are more often osteolytic.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree