14 Developmental Venous Anomaly
14.1 Introduction
Developmental venous anomalies (DVAs) are the most common vascular anomalies of the brain. They are most often discovered incidentally given the widespread use of MRI. Although they are rarely symptomatic, hemorrhage may occur, which was erroneously attributed to the DVA prior to the advent of MRI. It is now known that an associated cavernous malformation is the likely culprit for cerebral hemorrhage. Therefore, when hemorrhagic complications are encountered in the context of a known DVA, a thorough search for an associated cerebral cavernous malformation must be undertaken. The epidemiology, pathology, characteristic imaging features, and pertinent clinical issues including workup and management of DVAs are addressed.
14.2 Case Presentation
A 29-year-old Hispanic woman presented to a primary care clinic complaining of burning sensation to the right side of her head. The patient had no associated symptoms such as headaches, vision changes, or other neurologic complaints. On examination, she was neurologically intact. The patient was referred to our outpatient imaging facility.
14.3 Imaging Analysis
14.3.1 Imaging Findings
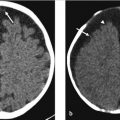
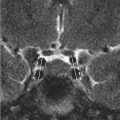
Initial postcontrast CT showed linear enhancing structures in the right frontal lobe (▶ Fig. 14.1). The smaller enhancing structures represented by dilated small veins are radially arranged and converge centripetally and drain into a larger venous structure (the “collector vein”; see ▶ Fig. 14.2). This pattern, described as the “caput medusae,” is the characteristic appearance of a DVA.


No additional imaging was recommended in this case.
14.3.2 Impression
Typical developmental venous anomaly, also known as venous angioma.
14.4 Clinical Evaluation
No follow-up is recommended for an incidental, asymptomatic DVA without associated cavernous malformation.
14.5 Differential Diagnosis
None. The classic caput medusae pattern is diagnostic of a DVA. 2
14.5.1 Diagnostic Imaging and Clinical Pearls
The caput medusae pattern is virtually diagnostic of a DVA. 2
DVAs are frequently incidental findings that are asymptomatic and follow a benign clinical course. 3
Prior to the advent of MRI, hemorrhage may have been wrongly attributed to DVAs. An associated vascular anomaly, most commonly a cavernous malformation, must be sought when hemorrhagic complications are seen.
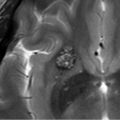
Susceptibility-weighted imaging (SWI) allows better visualization of DVAs without requiring contrast media 4 , 5 (▶ Fig. 14.3).


14.6 Essential Imaging Facts about DVAs
Perfusion imaging and pathophysiology:
Perfusion imaging allows qualitative and quantitative assessment of the flow characteristics throughout DVAs. 6
Venous congestion pattern is seen in larger DVAs with increased cerebral blood flow, cerebral blood volume, and mean transit time. 1 , 6 , 7
Abnormal perfusion parameters in some DVAs may indicate that they have less robust venous drainage capacity in comparison to normal brain. 7 , 8
DVAs may remain completely asymptomatic, suggesting that the diminished venous capacity is still sufficient to accommodate the physiologic needs of the territory drained. 8
Most importantly, the DVA drains normal brain and large venous infarctions often result from their surgical disruption. 7 Therefore, careful planning is needed when surgical intervention involves the territory of a DVA (i.e., cavernous malformation resection). 7
The term cerebral “developmental venous anomaly” was coined by Lasjaunias et al, 9 and it is recognized as a distinct clinical, radiological, and pathological entity. 10 , 11
DVA is a specific type of malformation of intracranial blood vessels that is composed exclusively of venous structures. 2 , 10 , 12
Conventional catheter angiography:
The diagnosis of DVAs has been historically based on their classic appearance on cerebral angiography. 1 , 13 , 14 “Caput medusae” describes a drainage pattern in which a myriad of small veins arising at the periphery converge into a larger central vein (the “collector vein”). 2 The collector vein follows a transcerebral course to reach a cortical surface, where it ascends to empty into larger cortical veins or a dural sinus. 2 , 14 Alternatively, the collector vein may drain into a subependymal vein in the wall of a lateral ventricle. 2
On angiogram, the dilated veins appear on the venous phase, and the collector vein persistently opacifies until the late venous phase. 11 , 14 , 15 DVA shows a normal circulation time and a normal arterial phase. 16
The majority of DVAs drain into the superficial venous system (70%), followed by deep drainage (20%), and less commonly a combination of both superficial and deep drainage. 9 , 11 , 17 They drain normal cerebral tissue within a functionally normal arterial territory. 9 , 16
Most importantly, DVAs are associated with absence of a venous pathway that would normally drain the territory. 9 , 16 , 18
On contrast-enhanced CT or MRI, DVAs appear as numerous linear and/or punctate enhancing foci converging on a well-delineated tubular collector. 1 , 7 , 11
14.7 Companion Cases
14.7.1 Companion Case 1
A 37-year-old man presented to the emergency center (EC) with headaches. On examination, the patient was neurologically intact with no focal deficits.
Imaging Findings
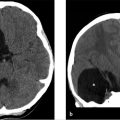
Initial noncontrast head CT in the EC revealed an acute parenchymal hematoma in the right parietal lobe with localized surrounding edema.
Additional Imaging in Companion Case 1
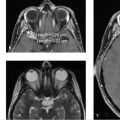
Further workup was undertaken to elucidate any underlying vascular etiology for the right parietal lobe hematoma. CT angiogram (CTA; ▶ Fig. 14.5a), MRI (▶ Fig. 14.5b), and catheter angiogram (▶ Fig. 14.6 and ▶ Fig. 14.7) were negative. However, an incidental DVA was found in the left frontal lobe, which did not have any associated parenchymal abnormality.



Follow-up Imaging in Companion Case 1

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree