Dermatomyositis
Gaucher’s Disease
Heavy Metal Poisoning
Histiocytosis
Hypertrophic Osteoarthropathy
Infantile Cortical Hyperostosis
Mastocytosis
Neurofibromatosis
Paget’s Disease
Scoliosis
Amyloidosis
BACKGROUND
Amyloidosis is a rare systemic disease caused by the extracellular accumulation of insoluble amyloid proteins in various organs and tissues of the body. Although several classifications exist, the simplest divides amyloidosis into primary and secondary forms. Amyloidosis without associated antecedent or coexisting disease is primary (idiopathic). Secondary amyloidosis is associated with chronic systemic disease (e.g., rheumatoid arthritis, Crohn’s disease, cystic fibrosis, chronic drug abuse), infections (e.g., familial Mediterranean fever, tuberculosis), and tumors (e.g., multiple myeloma). Usually amyloidosis is systemic; only 10% to 20% of cases demonstrate localized disease. 120 Primary amyloidosis affects men more than women and typically occurs between the ages of 40 and 80 years. The presentation of secondary amyloidosis depends on the associated underlying disorder.
IMAGING FINDINGS
Amyloidosis demonstrates a wide spectrum of imaging findings because a variety of systems, including the musculoskeletal, genitourinary, gastrointestinal, and cardiovascular systems, may be involved. 48,116
Osteonecrosis may develop from vessel occlusion after amyloid accumulation around capillaries and endothelial cells of larger blood vessels. Other findings include osteolytic bone destruction, periarticular joint swelling, osteoporosis, pathologic vertebral fractures, joint subluxations (e.g., proximal femur and humerus), and coarse trabeculae. Calcification may be seen in the amyloid deposits.
CLINICAL COMMENTS
The clinical presentation depends on which systems are involved. Amyloid deposits in the heart produce pleural effusion and dyspnea. Gastrointestinal involvement is associated with bowel obstruction and malabsorption. Localized amyloid deposits in the upper respiratory system may cause hoarseness and dysphagia. Definitive diagnosis requires biopsy confirmation; imaging studies are nonspecific.
• Amyloidosis results from localized or systemic protein deposits, known to involve a variety of systems.
• It occurs as a primary disease or secondary to multiple myeloma, rheumatoid arthritis, Crohn’s disease, and other systemic disorders.
• Associated bone changes include osteonecrosis, osteolytic destruction, periarticular joint swelling, and pathologic vertebral collapse.
Dermatomyositis
BACKGROUND
Inflammatory myopathies represent a group of disorders involving chronic inflammation, weakness, and wasting of skeletal muscle tissue. Inflammatory cells surround and destroy muscle fibers by a probable autoimmune-mediated mechanism. 86 Dermatomyositis, polymyositis, juvenile myositis, and inclusion body myositis all represent inflammatory myopathies. 85
Dermatomyositis represents chronic inflammation of the skeletal muscle and skin, affecting 5 out of every 10,000 people. It is the most easily recognized inflammatory myopathy resulting from the presence of a distinctive reddish rash occurring over the eyelids, cheeks, and nose. It occurs in all ages but is most common in adult women. Dermatomyositis is more common in children than are other myopathies. It is associated in adults with an elevated incidence of visceral carcinomas that increases with age. 6,103,104
IMAGING FINDINGS
The presence of subcutaneous calcifications is the most striking radiographic feature of dermatomyositis. Subcutaneous calcifications appear most commonly as linear or curvilinear radiodensities around the knees, elbows, and fingers (Fig. 15-1). Widespread calcifications (calcinosis universalis) develop in a few cases, severely limiting mobility. Calcification of intramuscular septa occurs in the deep muscles of the proximal limbs.
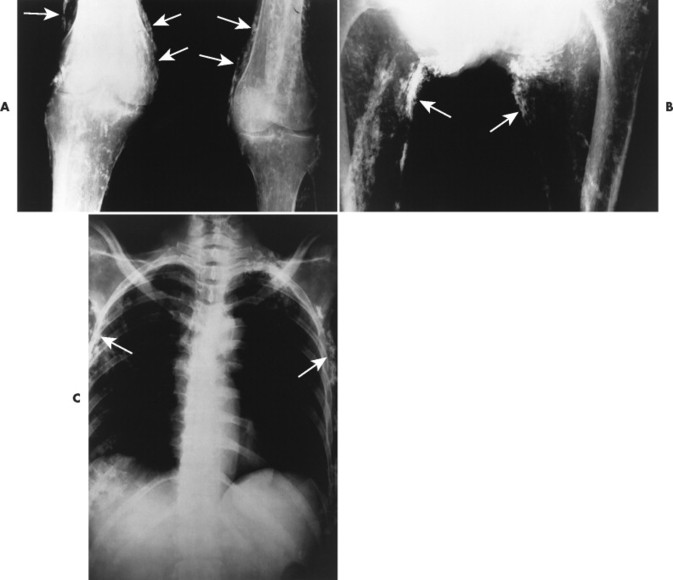 |
| FIG. 15-1 Dermatomyositis presenting as linear cutaneous calcifications (arrows) of, A, the knees, B, thighs, and C, thorax. (Courtesy Joseph W. Howe, Sylmar, CA.) |
Dermatomyositis is associated with arthritis primarily in the small joints of the hands, appearing with misalignment and juxtaarticular osteoporosis and soft-tissue swelling. Particularly characteristic is the “floppy thumb” sign, indicating subluxation of the interphalangeal joint of the first digit. A diffuse interstitial pattern is often present on the chest radiograph.
Magnetic resonance imaging (MRI) is helpful in localizing focal inflammatory myopathy, muscle atrophy, and fatty replacement of muscle. 87 MRI signal intensity correlates to the activity and distribution of the disease processes.
CLINICAL COMMENTS
Dermatomyositis often is not immediately painful; it may become noticeable only after muscle weakness and atrophy occur. The weakness may interfere with basic movements such as walking and raising arms. The diagnosis is made after an analysis of blood enzyme levels, electromyography, and muscle biopsy.
Steroids (e.g., prednisone) and immunosuppressive drugs (e.g., azathioprine) are given in an attempt to limit inflammation. 41 Passive range of motion and ice are indicated during acute exacerbations. Moderate exercise is advocated beyond the initial inflammatory stages. Muscle stretching appears to limit limb contracture.
The course of the disease is extremely variable, with some rapidly progressing to muscle wasting and weakness in just days, whereas others take years to progress to this stage. Disease complications include acute renal failure and malignancy.
• Dermatomyositis is a chronic inflammation of the skeletal muscle and skin, probably an autoimmune mechanism.
• Malignancy occurs with a higher incidence in adults. Dermatomyositis occurs in people of all ages but is most common in adult women.
• Dermatomyositis presents as a reddish rash occurring over the eyelids, cheeks, and nose.
• Linear or curvilinear subcutaneous calcifications may be found, primarily around the knees, elbows, and fingers.
• Misalignment, effusion, and osteoporosis of small joints of the hand accompany this disease.
• Standard treatment entails steroids, immunosuppressive drugs, passive range of motion, moderate exercise, and muscle stretching.
BACKGROUND
Gaucher’s disease is a lipid storage disorder resulting from a genetic deficiency of the enzyme glucocerebrosidase (glucosylceramidase), resulting in glucocerebroside accumulations within the cells of the reticuloendothelial system. 78 Gaucher’s disease is the most common hereditary metabolic storage disorder. 15
The disease may develop in individuals of any age, but it is more severe in children and especially infants. A higher incidence of the disease is seen in individuals who have Ashkenazi ancestry. 15
IMAGING FINDINGS
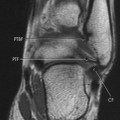
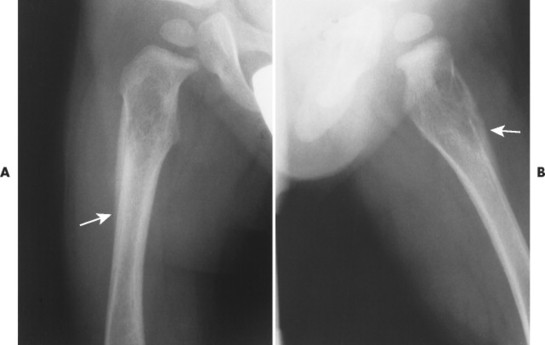
Accumulations of glucocerebroside may suppress bone marrow activity and cause destructive bone lesions and enlargement of the liver, spleen, and lymph nodes. The distal femur may exhibit thinned cortex and bone expansion (“Erlenmeyer flask” deformity). Single or multiple osteolytic lesions may occur and mimic the presentation of an infection or neoplasm (Fig. 15-2). Osteonecrosis may result from vascular occlusion, which follows the increase in the reticulum component of bone marrow (Fig. 15-3). The femoral head, humeral head, and wrist are particularly susceptible. Similar changes may produce H-shaped vertebrae at multiple levels (Fig. 15-4). MRI is useful to assess the extent and activity of bone marrow involvement (Fig. 15-5). 10,55
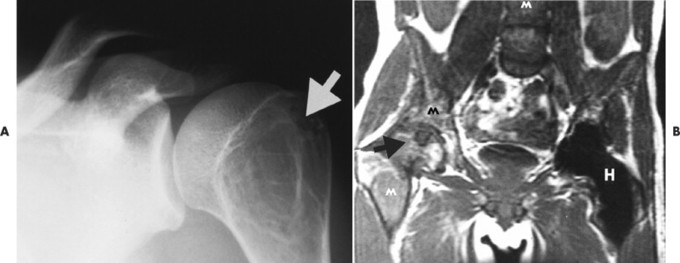 |
| FIG. 15-2 Gaucher’s disease. A, A lytic lesion in the proximal humerus (arrow) has resulted from a focal accumulation of lipid-laden cells. B, Coronal T1-weighted magnetic resonance imaging demonstrates diffuse low signal intensity, M, within the bone marrow, along with, H, osteonecrosis of the right femoral head (arrow) and contralateral hip replacement for the same process. The combination of findings is virtually diagnostic of the disease. (From Sartoris DJ: Musculoskeletal imaging: the requisites, St Louis, 1996, Mosby.) |
 |
| FIG. 15-3 Gaucher’s disease. Marrow infarct of the distal femora appearing as serpiginous calcifications in the distal femora (arrows). (Courtesy Steven P. Brownstein, MD, Springfield, NJ.) |
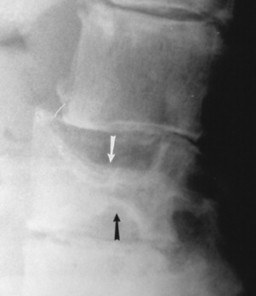 |
| FIG. 15-4 H-shaped vertebra in Gaucher’s disease. Central depression of the endplates (arrows) with sparing of the periphery is characteristic and occurs secondary to bone infarction. Differential diagnosis should include consideration of sickle-cell anemia and sickle-thalassemia. (From Sartoris DJ: Musculoskeletal imaging: the requisites, St Louis, 1996, Mosby.) |
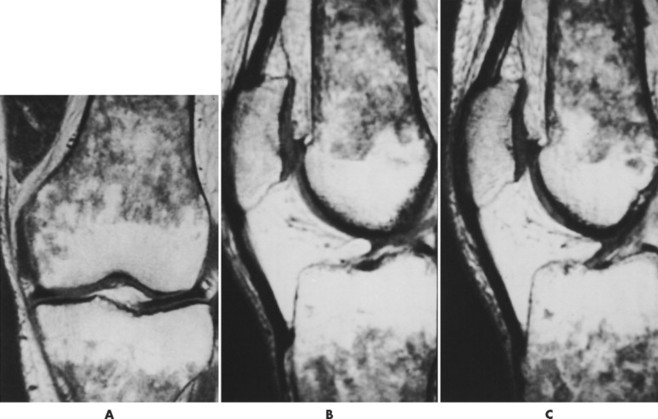 |
| FIG. 15-5 Gaucher’s disease in a 63-year-old man. Note the patchy decreased signal from the marrow of the distal femur and proximal tibia on, A, the coronal and, B and C, sagittal T1-weighted images. (From Firooznia H et al: MRI and CT of the musculoskeletal system, St Louis, 1992, Mosby.) |
CLINICAL COMMENTS
The clinical presentation of Gaucher’s disease is divided into three forms based on phenotype. 15 All three types are marked by hepatosplenomegaly and the presence of Gaucher’s cells in the bone marrow. Bone changes are more apparent in the chronic forms of the disease. The disease is more severe in infants because of cerebroside accumulations in neurons. All patients are predisposed to osteomyelitis. Intravenous infusion of glucocerebrosidase is an effective therapy but its application is limited because of its cost. 81
• Gaucher’s disease is a lipid storage disorder resulting from a genetic deficiency of the enzyme glucocerebrosidase; it is rare.
• Findings include anemia, hepatosplenomegaly, Erlenmeyer flask deformity, solitary or multiple osteolytic defects, osteonecrosis (usually femoral head), and H-shaped vertebrae.
Heavy Metal Poisoning
BACKGROUND
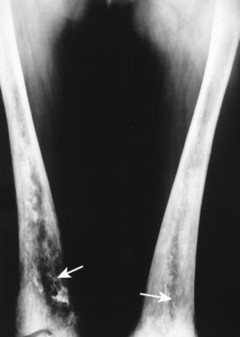
Poisoning may develop from the injection, implantation, ingestion, or inhalation of aluminum, bismuth, copper, lead, arsenic, phosphorus, mercury, or zinc. Lead is the most prevalent heavy metal, sometimes called the “silent epidemic.” Lead accumulates in the body over time, affecting multiple systems but principally the brain. Exposure is highest among occupations associated with extracting and processing lead, and among children who live in dilapidated housing whose exposure is related to the ingestion of peeling paint (Fig. 15-6). Lead-contaminated dust and soil are other potential threats of exposure for children. 74
 |
| FIG. 15-6 Lead intoxication. A, Radiodense bands in the ilium (arrowheads), proximal metaphyses of the femora (arrow), acetabuli (crossed arrow), and L5 vertebra secondary to lead intoxication. B, Another case of lead intoxication demonstrating mild radiodense lines in the acetabuli (arrow). Observe the radiodense ingested lead fragments in the gastrointestinal tract of the left upper abdominal quadrant (arrowhead). |
IMAGING FINDINGS
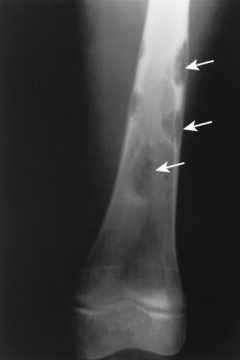
The most striking radiographic feature of lead, phosphorus, copper, or bismuth heavy metal poisoning is the presence of transverse radiodense lines in the metaphyses of long bones, especially around the knee. 11,128 The density of the bands is similar to that of cortical bone. Similar lines are noted with treated rickets, scurvy, and congenital syphilis, but they may also represent a normal variant. Copper, zinc, and aluminum toxicity are associated with altered bone mineralization and osteopenia.
Heavy metal poisoning is associated with a wide variety of nonspecific clinical findings, including anorexia, irritability, apathy, abdominal colic, vomiting, diarrhea, headaches, and convulsions. Toxic exposures damage the central nervous system, blood-forming organs, gastrointestinal tract, and other systems. 96,100
• Lead, phosphorus, copper, and bismuth poisoning are associated with transverse, radiodense metaphyseal bands.
• Copper, zinc, and aluminum toxicity are associated with altered bone mineralization and osteopenia.
Histiocytosis
BACKGROUND
Langerhans cell histiocytosis, formerly known as “histiocytosis X,” is the abnormal proliferation of histiocytes resulting in focal or systemic manifestations. Although the true etiology is not known, the prevailing opinion is that Langerhans histiocytosis represents a reactive rather than a neoplastic process. 126 Recently a better understanding of the disease has resulted from advances in specialized imaging and the development of immunohistochemical, morphologic, and clinical standards of diagnosis. 38
Langerhans cell histiocytosis describes three clinical syndromes: eosinophilic granuloma (60% to 80% of cases), Hand-Schüller-Christian disease (15% to 40% of cases), and Letterer-Siwe disease (10% of cases). Eosinophilic granuloma is the least aggressive form, usually seen in patients between the ages of 5 and 15 years.
Hand-Schüller-Christian disease is the chronic disseminated variety, characterized by multifocal bone lesions and extraskeletal involvement of the reticuloendothelial system. Typically it is seen in children between the ages of 1 and 5 years. Uncommonly (in 10% of cases) a clinical triad of exophthalmus, diabetes insipidus, and lytic skull lesions is present.
Letterer-Siwe disease represents an acute, disseminated, fulminant variety of the disease, seen in children younger than 2 years of age. Its aggressive clinical presentation may mimic leukemia. Most cases are fatal.
IMAGING FINDINGS
The osseous lesions of eosinophilic granuloma, Hand-Schüller-Christian, and Letterer-Siwe diseases are very similar. They appear as one or more medullary-based, lytic lesions with geographic destruction (Figs. 15-7 and 15-8), lobular contour, endosteal scalloping (Fig. 15-9), periosteal reaction (long bone lesions only) (Fig. 15-10), and well-defined, uneven, or beveled margins. 42 Matrix calcification and subarticular extension are not characteristic. 42 Alternatively, sclerotic lesions with or without periosteal reactions may occur.
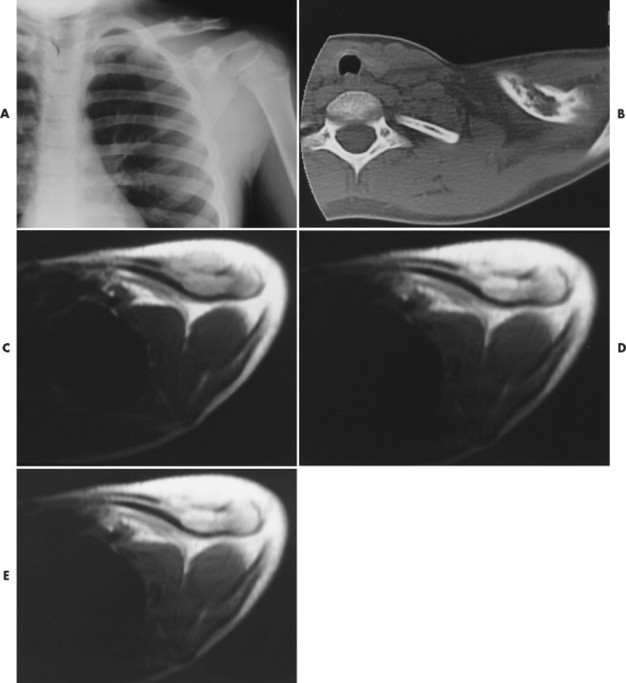 |
| FIG. 15-7 Eosinophilic granuloma in the left clavicle of a 9-year-old boy. A, Radiograph demonstrates a well-defined lytic defect. B, Computed tomography scan shows the lytic mass in the clavicle, with extension through the anterior cortex. C, Axial T1-weighted image depicts a well-defined, homogeneous mass that extends anteriorly through the cortex, with an associated anterior soft-tissue mass. D, Axial proton-density–weighted image shows a homogeneous mass of increased intensity, with a larger associated area of soft-tissue edema. Extension of soft-tissue edema along the shaft a considerable distance from the intramedullary lesion typically occurs with eosinophilic granuloma. E, The lesion brightens on the T2-weighted axial image. (From Stark DD, Gradley WG: Magnetic resonance imaging, ed 3, St Louis, 1999, Mosby.) |
 |
| FIG. 15-8 Several well-defined osteolytic regions secondary to eosinophilic granuloma (arrows). (Courtesy Joseph W. Howe, Sylmar, CA.) |
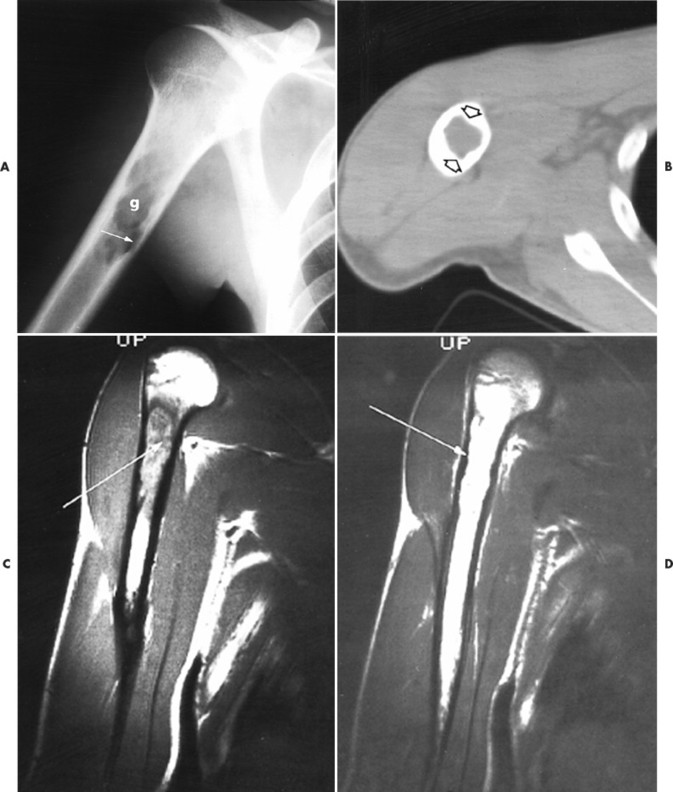 |
| FIG. 15-9 Eosinophilic granuloma. A, Radiography demonstrates, g, a well-defined lytic lesion in the proximal humeral diaphysis with endosteal scalloping (arrow). B, Computed tomography documents endosteal erosion (arrows) to better advantage. C, Coronal T1-weighted magnetic resonance image reveals predominantly low signal intensity (arrow) within the lesion. D, The lesion exhibits high signal intensity (arrow) on a corresponding T2-weighted image. Differential diagnosis should include consideration of fibrous dysplasia, plasmacytoma, metastatic disease, and indolent infection. (From Sartoris DJ: Musculoskeletal imaging: the requisites, St Louis, 1996, Mosby.) |
 |
| FIG. 15-10 A, Anteroposterior and, B, frog-leg projections of an aggressive osteolytic lesion in the proximal femur resulting from eosinophilic granuloma. Also noted is parallel periostitis of the lateral femur (arrows). (Courtesy Joseph W. Howe, Sylmar, CA.) (Courtesy Joseph W. Howe, Sylmar, CA.) |
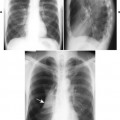
Overall, more than 50% of osseous lesions occur in the flat bones of the skull, pelvis, and ribs; about 30% of lesions occur in long bones (Fig. 15-11). 114 Spinal involvement usually spares the vertebral arch and may lead to advanced vertebral collapse (vertebral plana). As the lesions heal, the diminished vertebral height may reconstitute (Fig. 15-12). Soft-tissue masses may occur, representing extension from adjacent bone marrow involvement. 58 Advanced bony destruction of the mandible produces an isolated, “floating” appearance of the teeth. Pulmonary manifestation of eosinophilic granuloma includes a reticulonodular pattern of the middle and upper lung zones, often progressing to honeycomb lung.
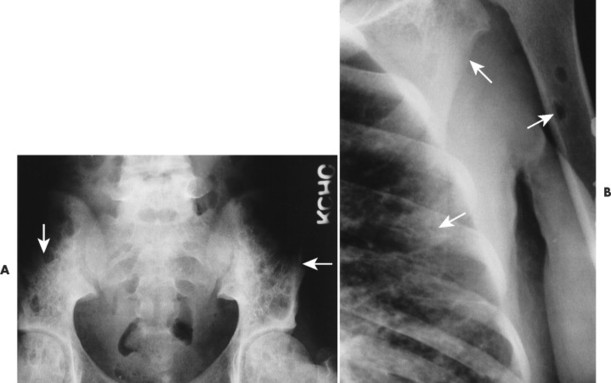 |
| FIG. 15-11 A, Eosinophilic granuloma marked by multiple osteolytic defects causing a cystic appearance to the pelvis (arrows). B, The same patient exhibits two well-defined osteolytic changes in the proximal humerus, ribs, and scapula (arrows). (Courtesy Steven P. Brownstein, MD, Springfield, NJ.) |
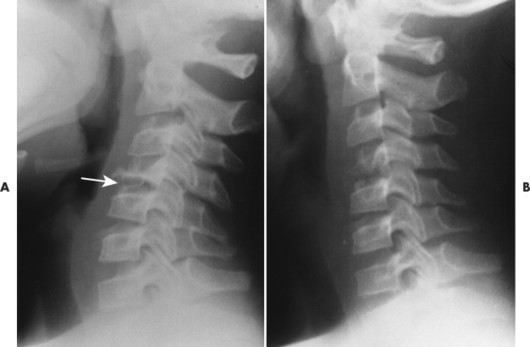 |
| FIG. 15-12 A, Eosinophilic granuloma leading to flattened C4 vertebral body (arrow). B, On a later film, the C4 vertebral body has partially reconstituted. (Courtesy Steven P. Brownstein, MD, Springfield, NJ.) |
Osseous lesions occurring with Letterer-Siwe disease typically are limited to the skull, often appearing as widespread and multiple osteolytic lesions. Eosinophilic granuloma typically presents as a solitary lesion and involves the appendicular skeleton more often than Letterer-Siwe or Hand-Schüller-Christian disease. Each of the three types, especially Letterer-Siwe, may appear permeative with widespread bone destruction, mimicking findings of leukemia, Ewing’s sarcoma, or infection. In general, Langerhans histiocytosis has such a variable appearance that it should be considered in every destructive bone lesion that appears in patients younger than 30 years of age.
Although radionuclide scintigraphy is more sensitive than radiographic skeletal surveys in detecting histiocytic lesions in the spine, pelvis, and ribs, it is less sensitive in identifying lesions in the skull. 35
CLINICAL COMMENTS
Pain, fever, elevated erythrocyte sedimentation rate, progressive anemia, hepatosplenomegaly, lymphadenopathy, and diabetes insipidus may be present. 33 The clinical presentation may mimic an infection. Overall, the prognosis of Langerhans cell histiocytosis is excellent in children with either localized or multifocal disease occurring in the absence of organ dysfunction, chronic disease, new-onset pituitary involvement, or long-term pulmonary fibrosis. 1,76 Current therapeutic approaches involve chemotherapies, immunosuppressives, bone marrow transplant, and gene therapy. 1
• Langerhans cell histiocytosis is marked by an abnormal proliferation of histiocytes.
• Langerhans cell histiocytosis lesions demonstrate a wide variety of osseous presentations and should be considered as a diagnostic differential in every destructive bone lesion occurring in patients younger than 30 years of age.
• Hand-Schüller-Christian disease, which occurs in 15% to 40% of these cases, is the chronic disseminated variety occurring in children between 1 and 5 years of age. Osseous lesions typically are multiple geographic osteolytic lesions, occurring in the skull, pelvis, and long bones.
• Eosinophilic granuloma (which occurs in 60% to 80% of cases) is the least aggressive form, usually seen in patients between the ages of 5 and 15 years. Osseous lesions usually are solitary geographic osteolytic lesions, occurring in the skull, pelvis, long bones, mandible, and spine.
Hypertrophic Osteoarthropathy
BACKGROUND
Hypertrophic osteoarthropathy describes a primary (pachydermoperiostosis or Touraine-Solente-Golé syndrome) or secondary (Pierre-Marie-Bamberger syndrome) disorder accompanied by digital clubbing, painful swollen joints, and a symmetric, undulated, periosteal reaction. 9,72 All features of the disorder may not be present. Although the etiology remains elusive, vascular flow, vascular endothelium, and platelet-derived growth factors have all been implicated. 32,63,71 The primary form is less common (occurring in 3% to 5% of cases), has an adolescent onset and a predominance for males and blacks, and is associated with a thickened appearance of the skin of the face and scalp.
Secondary hypertrophic osteoarthropathy is associated with bronchogenic carcinoma (which occurs in up to 12% of cases), 125 pulmonary abscess, pulmonary metastasis, Hodgkin disease, 89 emphysema, cystic fibrosis, heart disease, and occasionally in other acute and chronic disorders. 21,111 Lesions of the abdominal cavity (e.g., dysentery, Crohn’s disease, biliary atresia) may produce secondary hypertrophic osteoarthropathy; however, intrathoracic causes predominate, especially bronchogenic carcinoma. The age of onset is related to the underlying pathology.
IMAGING FINDINGS
The radiographic features of primary and secondary hypertropic osteoarthropathy are similar. Both are marked by periosteal reaction occurring most commonly in the tubular bones of the extremities, especially the tibia, fibula, radius, and ulna. Less commonly the wrist, ankle, and small bones of the hands and feet are involved. The periosteal reaction is nonaggressive, thick, and widespread, occurring in the diaphysis and metaphysis (Fig. 15-13). The thickness of the periosteal reaction may increase proportionally to the duration of the disease.
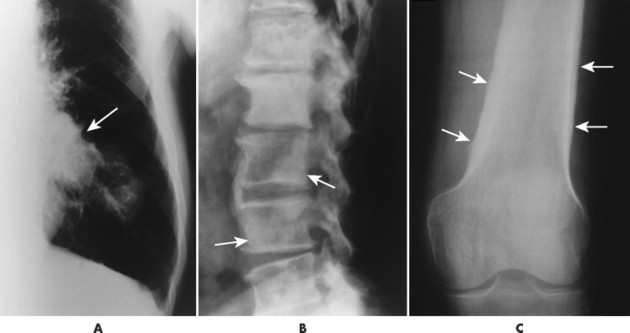 |
| FIG. 15-13 A, Bronchogenic carcinoma of the lung (arrow) has metastasized to, B, the lumbar spine (arrows) and manifested with hypertrophic osteoarthropathy by producing thick periostitis along, C, the distal femora (arrows). (Courtesy Joseph W. Howe, Sylmar, CA.) |
The differences between the primary and secondary forms are that the periosteal reaction occurring in patients with primary hypertrophic osteoarthropathy involves the epiphyses (secondary does not) and appears more “fluffy,” “shaggy,” or less well defined than does the reaction associated with secondary osteoarthropathy. Ligamentous calcifications and bony excrescences are features of primary hypertrophic osteoarthropathy, often involving the calcaneus, patella, and interosseous membrane between the radius and ulna.
CLINICAL COMMENTS
The clinical onset of primary hypertrophic osteoarthropathy is insidious, marked by clubbing of the distal hands and feet. The skin of the face and scalp appears thickened (pachydermia). The clinical presentation of secondary osteoarthropathy is similar but also is dependent on the underlying condition. A clinical presentation of vague bone pain and joint swelling occurs more commonly in the secondary form of the disease. The primary form usually is self-limiting after many years of involvement.
• Primary hypertrophic osteoarthropathy is marked by a solid, thick, shaggy periosteal reaction involving the epiphysis, metaphysis, and diaphysis of the tibia, fibula, radius, and ulna. Clubbing of the fingers, ligamentous calcification, and skin changes of the scalp and face are noted.
• Secondary hypertrophic osteoarthropathy occurs secondary to underlying disease (usually bronchogenic tumor) and demonstrates a solid, thick, well-defined periosteal reaction involving the metaphysis and diaphysis of the tibia, fibula, radius, and ulna. Clubbing of the fingers and joint swelling are common clinical features.
BACKGROUND
IMAGING FINDINGS
The radiographic features include a symmetric, thick periosteal reaction most commonly involving the mandible (80% of cases), clavicle, ulna, and less commonly the ribs, scapulae, calvarium, or diaphysis of tubular bones (Fig. 15-14). 45 Although the mandible is the most commonly affected bone in the sporadic form, the tibia is the predominant bone affected in patients with the familial form. 14
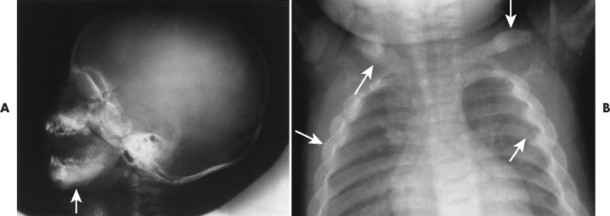 |
| FIG. 15-14 Infantile cortical hyperostosis (Caffey’s disease) presenting with bilaterally symmetric thick periostitis (arrows) of, A, the mandible clavicles and, B, ribs. (Courtesy Ian D. McLean, Davenport, IA.) |
Physiologic periostitis is a much more common cause of periosteal reaction in infants younger than 6 months of age. A similar appearance may occur in rickets and scurvy but rarely before 6 months of age, and each demonstrates additional metaphyseal findings. Pleural effusion may accompany rib involvement. Radionuclide scintigraphy may be useful to further delineate the extent of skeletal involvement. MRI is useful to assess the presence and extent of subperiosteal hemorrhage. 102
CLINICAL COMMENTS
The clinical manifestation is marked by fever, hyperirritability, soft-tissue swelling over the involved bone, and often an elevated erythrocyte sedimentation rate. On palpation, the masses of involved bone and soft-tissue swelling are hard and painful. The clinical course is typically self-limiting within a few months. 14
• Infantile cortical hyperostosis occurs before 6 months of age.
• Fever, hyperirritability, and painful soft-tissue swelling over affected bones are clinical symptoms.
• This disease most commonly involves the mandible and clavicle.
Mastocytosis
BACKGROUND
Mastocytosis is a term used collectively to describe a heterogeneous group of disorders all characterized by an abnormal proliferation and accumulation of mast cells in various tissues and organs. 112,121 The etiology is largely unknown, but limited evidence points to an abnormality of stem cell factor receptors. 44 Mastocytosis usually is limited to the skin (90% of cases), but rarely it may become systemic, involving primarily the bone marrow and gastrointestinal tract. 30,44,49 Systemic disease usually manifests in adults, although a pediatric form of systemic disease exists. 64 Men and women are affected equally.
IMAGING FINDINGS
The radiographic appearance of systemic mastocytosis is variable. Histamine and heparin released from the mast cells may produce generalized osteopenia similar to osteoporosis. 28,59 Localized osteopenic or osteolytic defects are observed less commonly. The osteolytic lesions occur most often in the ribs, skull, and long tubular bones. Mast cell proliferation and infiltration may cause reactive sclerosis of the host bone, producing multiple osteosclerotic foci. The osteosclerotic lesions are more common in the axial skeleton and may be accompanied by thickened trabeculae. The osteolytic and osteosclerotic regions often coexist (Fig. 15-15). Radionuclide bone scintigraphy may be helpful to determine the full extent of skeletal involvement.
 |
| FIG. 15-15 Mastocytosis causing increased radiodensity and a mottled appearance (arrows) in the lumbar spine and pelvis. (Courtesy Steven P. Brownstein, MD, Springfield, NJ.) |
CLINICAL COMMENTS
Systemic mastocytosis comprises a wide spectrum of clinical features, depending on the organs involved, age of onset, and associated hematologic diseases. 44 Clinical features are related to the release of mast-cell–derived mediators (e.g., heparin, histamine, platelet-activating factor, prostaglandin, peptide leukotrienes) and include vomiting, flushing, diarrhea, hepatosplenomegaly, weight loss, and skin lesions. 91 Treatment is directed toward symptom relief and the prognosis is dependent on the extent of involvement.
• Mastocytosis involves the rare proliferation of mast cells, typically affecting adults.
• This disease involves general or local osteopenia, local osteosclerosis, or a mixed pattern of presentation.
• The clinical symptoms of mastocytosis are vomiting, flushing, weight loss, and diarrhea.
BACKGROUND
Neurofibromatosis is the most common of a heterogeneous group of diseases known as phakomatoses. Phakomatoses are disorders of embryologic neuroectoderm tissue derivatives and are characterized by hamartomas of various tissues. Other phakomatoses include Lindau disease, Sturge-Weber syndrome, and tuberous sclerosis.
Neurofibromatosis is a genetic disorder affecting primarily the cell growth of neural tissue. 80 At least eight presentations of the disease exist; however, only two are widely recognized: neurofibromatosis type I (von Recklinghausen’s disease, peripheral neurofibromatosis, or NF-1) and neurofibromatosis type II (central neurofibromatosis, or NF-2). 109 Both types have an autosomal dominant pattern of inheritance, no sexual or racial predilection, and are marked by nerve sheath tumors. Each appears to be influenced by hormones, and women may notice exacerbations during pregnancy. Other than these common points, the two types appear very different clinically and reflect defects of two different genes (chromosome 17 in NF-1 and chromosome 22 in NF-2).
Neurofibromatosis type I.
NF-1 is the most common form of the disorder, affecting approximately 1 in 4000 individuals. 80 It is diagnosed when at least two of the following criteria are present:
1. Six or more cutaneous macules (café-au-lait spots), larger than 5 mm before puberty, larger than 15 mm after puberty
2. Two or more neurofibromas
3. One or more plexiform neurofibromas
4. Axillary or inguinal flecking
5. Optic gliomas
6. Two or more iris hamartomas (Lisch nodules)
7. One or more characteristic bone lesions
8. First-degree relative (parent, child, or sibling) with the disease81
NF-1 is associated with a variety of intracranial hamartomatous and neoplastic lesions of the white matter and globus pallidus, including optic nerve and parenchymal gliomas. 88 Those afflicted exhibit characteristic nonelevated brownish cutaneous hyperpigmentations, known as café-au-lait spots. The hyperpigmentations exhibit smooth margins (“coast of California”), as opposed to the jagged margins (“coast of Maine”) of similar hyperpigmentations that occur with polyostotic fibrous dysplasia. Other cutaneous lesions include the presence of multiple, widely dispersed soft nodules known as fibroma molluscum. In addition to the intracranial and cutaneous lesions, patients with NF-1 exhibit osseous defects of the skull, spine, and extremities, which are detailed under Imaging Findings. 51
Neurofibromatosis type II.
NF-2 is a much less common form of neurofibromatosis than NF-1, affecting 1 in 50,000 individuals. 80 It is diagnosed by the presence of bilateral acoustic schwannomas or a unilateral acoustic schwannoma occurring in a patient who has a first-degree relative (parent, child, or sibling) with the disease. 80 It is common for patients to develop unilateral acoustic schwannomas unrelated to neurofibromatosis. In addition to acoustic schwannomas, patients often develop schwannomas of other cranial nerves, and solitary or multiple meningiomas. 115 In contrast to NF-1, cutaneous and osseous changes are not characteristic of NF-2.
IMAGING FINDINGS
Plain film radiography offers little in the evaluation of the intracranial manifestations of NF-1 or NF-2, although many of the skeletal changes occurring with NF-1 are clearly seen on plain film radiography. Rarely an enlarged internal acoustic or optic canal may be seen, but these osseous changes always are delineated better with computed tomography (CT). MRI offers superb imaging of intracranial and spinal lesions (Fig. 15-16). Patients with NF-1 often show hyperintense foci in the areas of brain involvement on T2-weighted images. The intensity of these areas varies over serial studies. Neurofibromas often demonstrate a character-istic pattern of peripheral hyperintensity and central hypointensity on T2-weighted images (target sign). The intracranial lesions of NF-2 (acoustic schwannomas and less frequently associated meningiomas) also are imaged best by MRI. On MRI film, schwannomas are hypointense or isointense relative to brain parenchyma on T1-weighted scans, are hyperintense on T2-weighted scans, and enhance after gadolinium. 95
 |
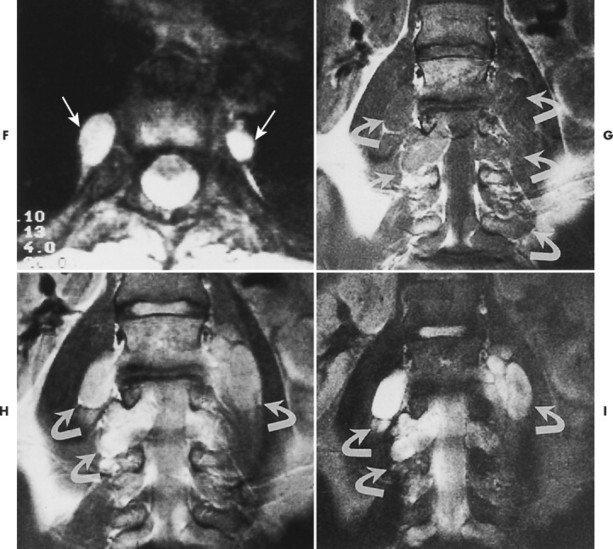 |
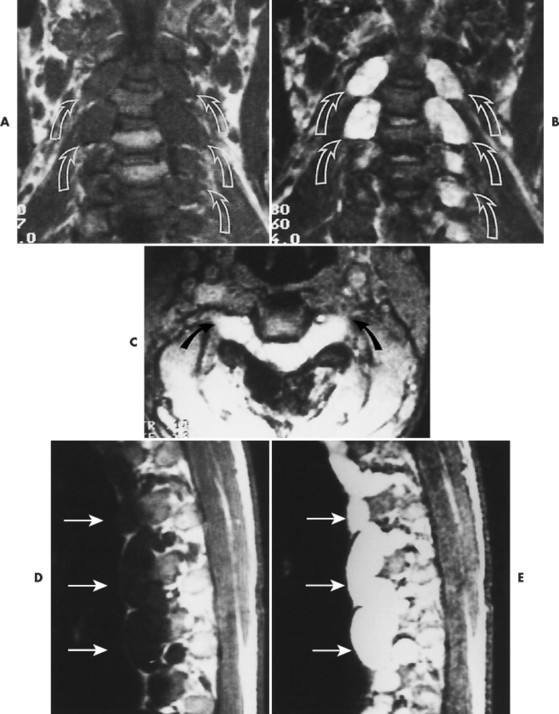
| FIG. 15-16 Multiple soft-tissue masses emanating from the spinal canal in a young patient with a known history of neurofibromatosis. A, A T1-weighted coronal image through the cervical spine shows that the masses are both intradural and extradural (arrows). B, A T2-weighted image through the same region demonstrates increased signal within the lesions. Some have ill-defined areas of decreased signal, characteristic of neurofibromas (arrows). C, A 10-degree FISP gradient echo (fast imaging with steady-state precession) image shows widening of the cervical neural foramina by the high-signal lesions (arrows). D, A T1-weighted parasagittal image through the thoracic spine reveals multiple septate cavities just lateral to the canal (arrows). E, Homogeneously increased signal consistent with a thoracic meningocele (arrows). F, The 10-degree gradient echo axial study again shows the lateral meningocele (arrows). G, A T1-weighted coronal image through the lumbar spine demonstrates multiple intradural and extradural “dumbbell” neurofibromas that have the signal intensity of soft tissue (arrows). H, A proton-density–weighted coronal scan through the lumbar spine shows the dumbbell shape of the neurofibromas, which now are of increased signal intensity (arrows). I, A T2-weighted coronal scan through the lumbar spine reveals the high-signal neurofibromas, some of which have a lower signal intensity within (arrows). (From Modic et al: Magnetic resonance imaging of the spine, St Louis, 1994, Mosby.) |
Skull.
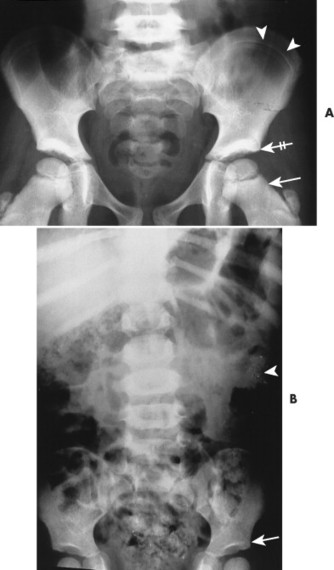
Congenital deficiency of the greater and lesser wing of the sphenoid and posterosuperior orbital wall creates a “bare orbit” appearance (Fig. 15-17). This abnormality allows the temporal lobe of the brain to directly contact the soft tissues around the eye, producing pulsatile exophthalmus. 20 Other changes include a radiolucent cranial defect at the lambdoidal suture (asterion defect) just posterior to the junction of the parietomastoid and occipitomastoid sutures (Fig. 15-18). This defect is more common on the left side and may be associated with ipsilateral hypoplasia of the mastoid. Enlargement of cranial foramen may be present, especially of the optic and internal auditory canals. Enlargement of the entire skull (macrocranium) is a common and prominent feature in up to 75% of individuals. An additional finding may be the presence of scattered calcifications over the temporal lobe similar to those that occur in the choroid plexus. 130
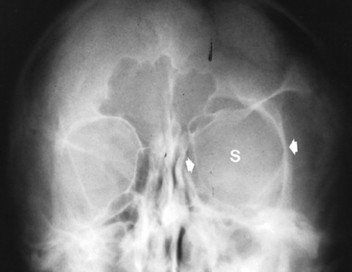 |
| FIG. 15-17 Unilateral absence of the sphenoid wing (s) with orbital enlargement (arrows) in neurofibromatosis. The findings are virtually specific for this condition and occur secondary to mesodermal dysplasia. (From Sartoris DJ: Musculoskeletal imaging: the requisites, St Louis, 1996, Mosby.) |
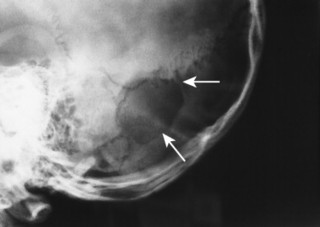 |
| FIG. 15-18 Neurofibromatosis with lambdoidal suture (asterion) defect (arrows). |
Spine.
The spine is affected commonly. 67,129 Kyphoscoliosis is seen in 50% of patients and is marked by a short segment; angular, progressive curvature most often occurs in the thoracic spine. The kyphoscoliosis may lead to paraplegia. Scalloping of the posterocentral portion of the vertebral body is related to dural ectasia. Neurofibromas of the exiting nerve roots may cause scalloping defects of the posterior vertebral body and other borders of the corresponding intervertebral foramen (FIG. 15-19FIG. 15-20FIG. 15-21FIG. 15-22 and FIG. 15-23). Large scalloped osseous defects and paraspinal masses occur with intrathoracic meningoceles, which represent lateral protrusions of the spinal canal’s meninges into the extrapleural space.
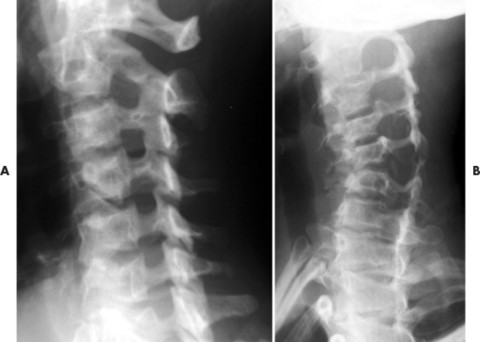 |
| FIG. 15-19 A, Normal oblique projection demonstrating the normal appearance of the cervical intervertebral foramen. B, Case of neurofibromatosis presenting with enlargement of the cervical intervertebral foramen resulting from pressure erosions of the neurofibromas contained within the foramen. One or more levels of enlarged intervertebral foramen are typical of the disease. |
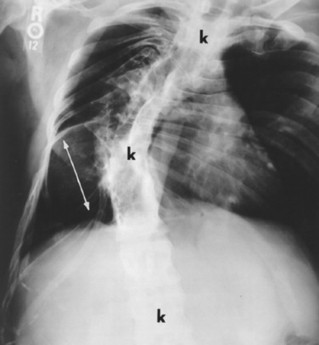 |
FIG. 15-20
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|