2 Brain
Approach
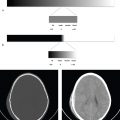
Noncontrast head CT is one of the most frequently ordered emergency studies. Common indications include headache, suspected cerebral infarct or intracerebral hemorrhage, altered mental status, and trauma.
Images should always be evaluated with windows and reconstruction algorithms optimized for brain, subdural hematoma, and bone. In trauma, multidetector CT from the skull base to the upper thoracic spine can be acquired to image the head, cervical spine, face, and skull base in a single scan. Thin-section reconstructions and reformations in multiple planes or three-dimensional surface renderings can then be extracted from the imaging data obtained.
Noncontrast CT is the initial study for detection of hemorrhage, infarct, intracranial masses, traumatic injuries, and assessment of overall brain volume and quality. A normal noncontrast CT excludes most emergent and surgical conditions. MRI with gadolinium is more sensitive for detection of infectious or neoplastic conditions including metastases, cerebritis, meningitis, and leptomeningeal neoplasm, but it is not typically necessary for acute management. Contrast CT is an alternative if MRI is not available or contraindicated in a particular patient.
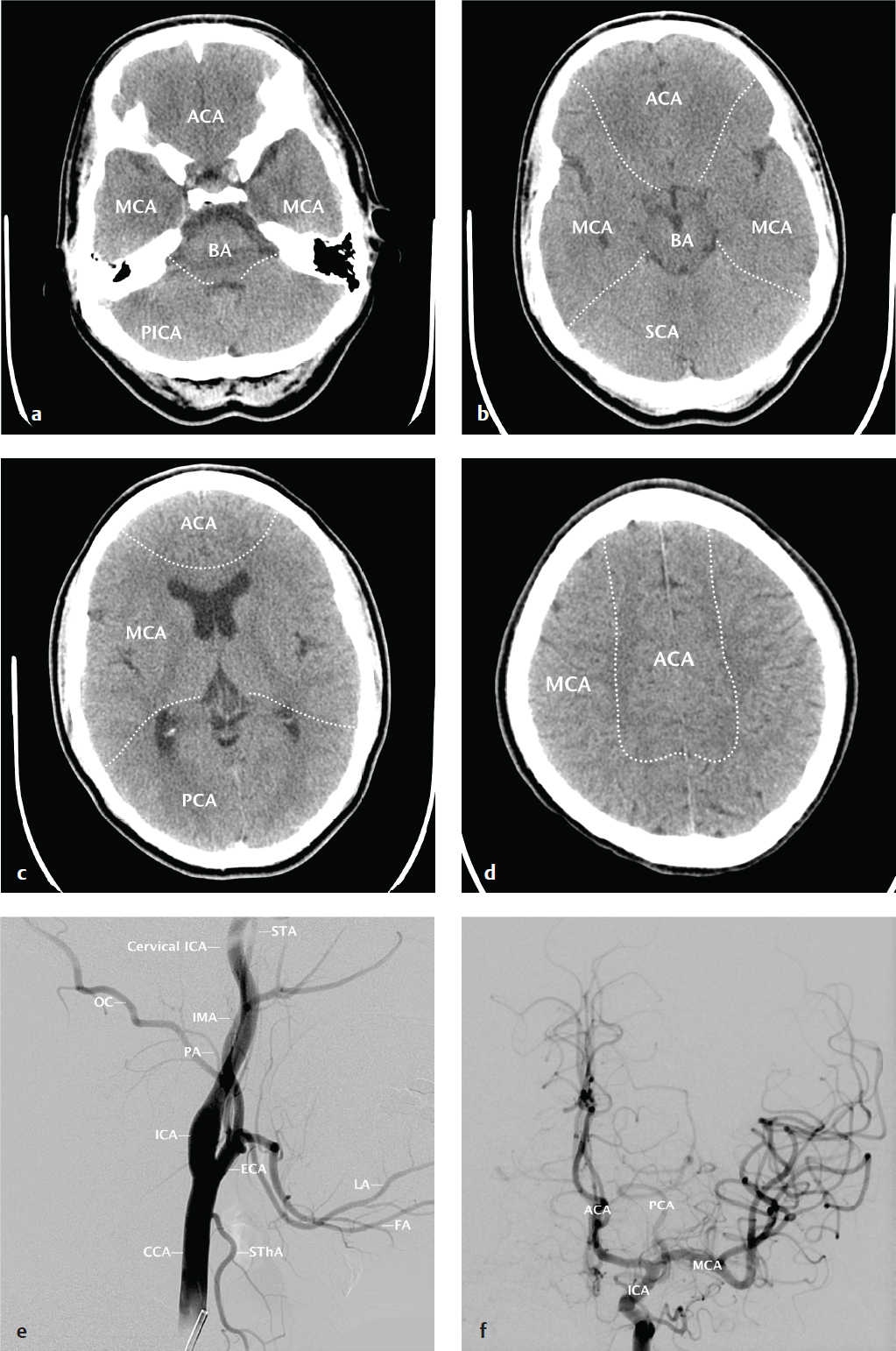
Cerebral CT angiography, in which images are obtained during the arterial or early venous phase of contrast enhancement, has largely replaced catheter angiography for primary detection of cerebral aneurysms, arteriovenous malformations (AVM), vascular injuries, and venous or cavernous sinus thrombosis. Conventional angiography is usually reserved for preoperative aneurysm or AVM evaluation, and endovascular therapy. CT perfusion imaging can be useful in identifying the location and extent of vascular compromise in patients with acute stroke symptoms.
On older scanners, brain CT is usually obtained nonhelically to avoid artifacts. Newer multislice CT systems permit adequate imaging of the brain in three planes as well as simultaneous acquisition of high-resolution images of the face and cervical spine in patients with acute head and neck trauma.
Checklist
Scalp
Skull
Epidural space (potential)
Subdural space (potential)
Dural sinuses and reflections
Cortex
Subcortical white matter
Basal ganglia
Ventricles
Cisterns
Cerebral vessels
Pituitary and sella turcica
Skull base
Orbits
Sinuses
Facial bones
Imaging and Anatomy
Imaging
Head CT (Noncontrast)
Indications: Head injury, altered mental status, seizure, suspected hemorrhage or infarct.
Technique: 5-mm axial images in soft tissue and bone algorithm
Head CT (Noncontrast Helical)
Indications: Head injury with concurrent imaging of the face and cervical spine.
Technique: Helical 0.6-mm dataset with 5-mm axial, 2-mm sagittal, and 2-mm coronal reformations of head, face, and cervical spine. Images obtained from skull vertex to thoracic inlet.
CT Arteriogram
Indications: Subarachnoid hemorrhage. Suspected aneurysm or vascular malformation. Acute cerebral infarct. Penetrating injury.
Technique: Helical 0.6-mm dataset with 2.5-mm axial, 2-mm sagittal, and 2-mm coronal reformations. Images can be obtained from the vertex either to the skull base or to the thoracic inlet depending on the indication.
Contrast: 60–100 mL at 3–4 mL/sec in arterial phase.
CT Venogram
Indications: Suspected venous sinus thrombosis (atypical headache). Trauma to skull base with potential venous sinus disruption.
Technique: Helical 0.6-mm dataset with 2.5-mm axial, 2-mm sagittal, and 2-mm coronal reformations. Images obtained from vertex to skull base.
Contrast: 60–100 mL at 3–4 mL/sec in venous phase (30–45 sec delay).
Anatomy
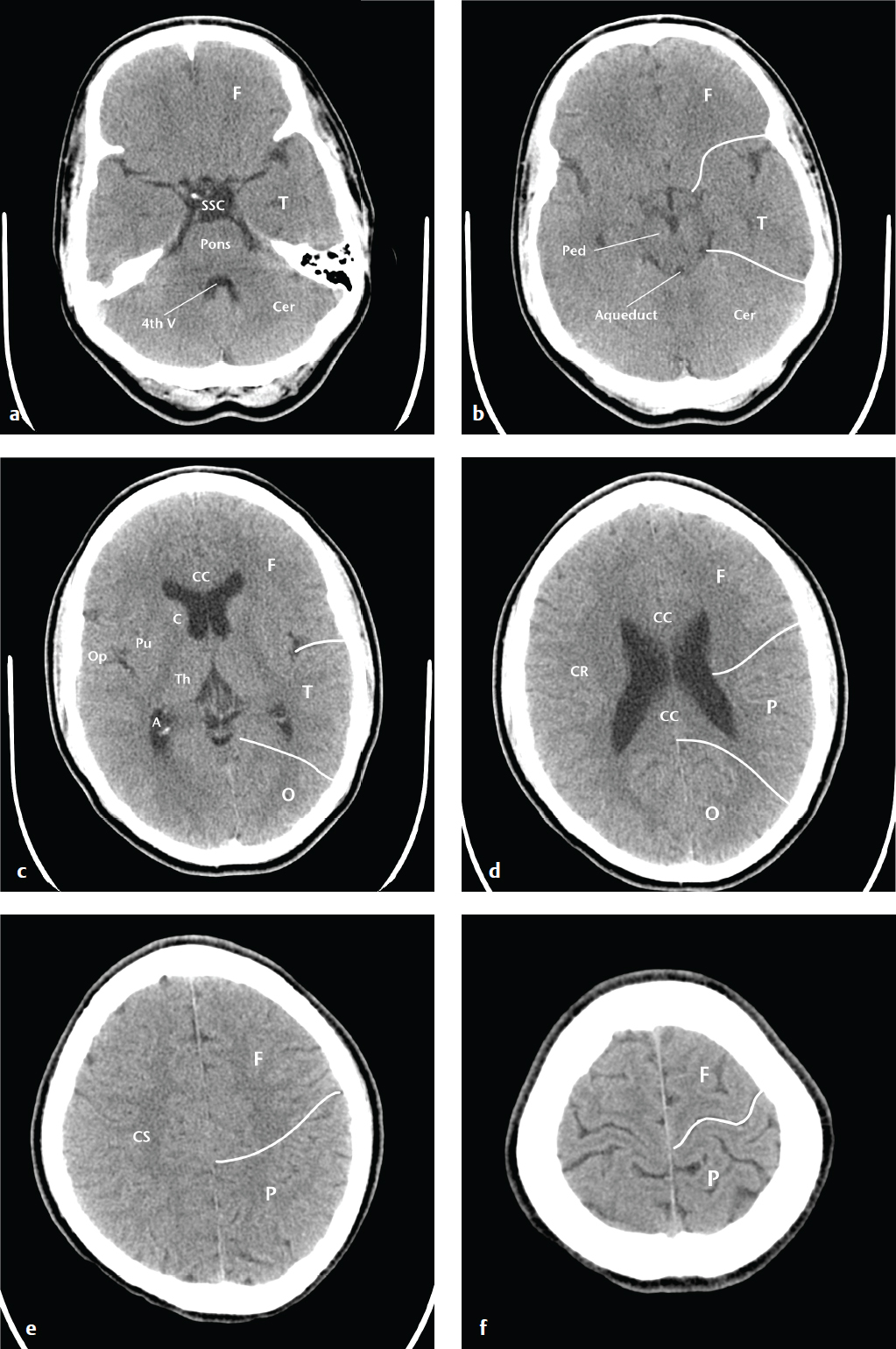
Effective description and analysis of cerebral pathology requires knowledge of visible cerebral structures (lobes, basal nuclei, ventricles), vascular territories, and arterial and venous anatomy. Cerebral anatomy is shown in Fig. 2.1 . Cerebral vascular territories and anatomy are seen in Fig. 2.2 .

Clinical Presentations and Differential Diagnosis
Clinical Presentations and Appropriate Initial Studies
Trauma
Noncontrast head CT is indicated. Head or neck CT angiography should be considered if injury mechanism or initial findings indicate a likely cervical vascular injury.
Skull fracture
Epidural hematoma
Subdural hematoma
Venous epidural hematoma
Traumatic subarachnoid hemorrhage
Contusion
Diffuse axonal injury
Skull fracture
Temporal bone fracture
Facial bone fracture
Headache
Noncontrast head CT is indicated. Postcontrast head CT or MRI may be considered in immunocompromised patients, in those with underlying malignancy and concern for metastatic disease, and in patients otherwise at risk for brain abscess.
Subarachnoid hemorrhage
Venous sinus thrombosis
Meningitis
Hydrocephalus
Cerebral hemorrhage
Mass (tumor or abscess)
Sinusitis
Otitis/mastoiditis
Acute Focal Neurologic Deficit
Noncontrast head CT is indicated to exclude hemorrhage in suspected stroke. Noncontrast head CT should be obtained to detect or exclude cerebral hemorrhage. MRI with a diffusion-weighted sequence can confirm the clinical suspicion of infarct in patients whose initial CT appears to be normal. CT or MR angiography and perfusion studies can be obtained to better characterize the extent of ischemia and identify vascular stenoses or occlusion.
Ischemic infarct
Lacunar infarct
Embolic infarct
Border-zone (watershed) infarct
Hypertensive hemorrhage
Amyloid angiopathy with hemorrhage
Ruptured arteriovenous malformation
Altered Mental Status
Noncontrast head CT is indicated. Post-contrast head CT or MRI may be considered in immunocompromised patients, in those with underlying malignancy and concern for metastatic disease, in patients otherwise at risk for brain abscess, and to evaluate further subtle abnormalities on noncontrast CT.
Cerebritis (herpes encephalitis)
Infarct
Hemorrhage
Parenchymal volume loss
Chronic subdural hematoma
Differential Diagnoses
Intraparenchymal Mass
Glioma
Metastasis
Lymphoma
Abscess
Arteriovenous malformation
Extraparenchymal Mass
Meningioma
Calvarial or dural metastasis
Epidermoid
Schwannoma (cranial nerves 5, 8, and 9)
Arachnoid cyst
Aneurysm
Sellar and Suprasellar Mass (Mnemonic: SATCHMO)
Sellar tumor (pituitary adenoma), sarcoidosis
Aneurysm
Teratoma (and germ cell tumors)
Craniopharyngioma
Hypothalamic glioma
Meningioma (planum sphenoidale) and metastasis
Optic glioma
Ring Enhancing Mass (Mnemonic: MAGIC DR)
Metastasis
Abscess
Glioma
Infarct (evolving)
Contusion (evolving)
Demyelinating disease
Radiation necrosis
Hemorrhagic Metastases (Mnemonic: CT/MR)
Choriocarcinoma
Thyroid carcinoma
Melanoma
Renal cell carcinoma
Remember that lung and breast carcinoma, even though less frequently hemorrhagic, are much more common and are more likely to be the cause of a hemorrhagic metastasis.
Parenchymal Hemorrhage
Hypertensive hemorrhage
Amyloid angiopathy
Hemorrhagic metastasis
Herpes encephalitis
Acute leukemia
Arteriovenous malformation
Aneurysm
Contusion (in trauma)
Subarachnoid Hemorrhage
Ruptured aneurysm
Benign perimesencephalic hemorrhage
Traumatic subarachnoid hemorrhage
Global Cerebral Volume Loss
Chronic alcohol use
Anticonvulsants (particularly cerebellar)
Dehydration
Steroid use
Chemotherapy
Remote head injury
Normal aging
Communicating Hydrocephalus
Normal pressure hydrocephalus
Meningitis
Prior subarachnoid hemorrhage
Venous sinus thrombosis
Obstructive Hydrocephalus
Aqueductal stenosis
Colloid cyst of third ventricle
Tectal glioma
Intraventricular tumor
Cerebral Edema Patterns
Localized cerebral edema may be classified as cytotoxic, vasogenic, or interstitial, although overlap is often present. Cytotoxic edema consists of intracellular swelling caused by depletion of adenosine triphosphate (ATP) and consequent sodium/potassium membrane pump failure. Most typical of cerebral infarct and anoxic injury, cytotoxic edema affects both white and gray matter similarly on most imaging studies.
Vasogenic edema, in contrast, preferentially involves white matter and results from conditions that increase intracerebral capillary permeability. Vasogenic edema is often due to brain contusion, severe hypertensive disease, primary and metastatic neoplasms, or inflammatory conditions.
Acute hydrocephalus can cause inter-stitial edema, as elevated intraventricular pressure leads to transependymal migration of CSF into the periventricular white matter, where it is resorbed via parenchymal capillaries.
On CT, cerebral edema is lower in density than surrounding normal parenchyma. On MRI, signal intensity of edema is lower than that of brain parenchyma on T1-weighted images and higher on T2-weighted images. Diffusion-weighted image (DWI) and apparent diffusion coefficient (ADC) mapping sequences can distinguish between cytotoxic edema (restricted diffusion) and vasogenic or interstitial edema (normal or increased diffusion). Fluid attenuation inversion recovery (FLAIR) sequences suppress intraventricular CSF signal and are sensitive for detecting adjacent areas of interstitial edema ( Fig. 2.3 ).
Anatomic Variants and Incidental Findings
Common incidental findings on head CT include arachnoid cysts, prominent arachnoid granulations, choroid plexus and choroidal fissure cysts, remote lacunar infarcts, focal encephalomalacia from prior infarct or trauma, and prominent perivascular spaces. The ventricles may be slightly asymmetric, and the septum pellucidum may contain a central CSF-filled cavity (cavum septum pellucidum). Unless symptomatic, these conditions usually do not require specific follow-up ( Fig. 2.4 ).
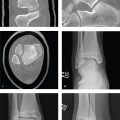
Skull Fracture
A calvarial fracture does not reliably predict underlying brain injury, and a normal skull radiograph does not exclude intracranial injury. Calvarial fractures that are depressed more than 5 mm are usually elevated surgically. Epidural hematoma is associated with fractures that traverse the course of the middle meningeal artery. Skull base fractures are associated with high-energy trauma and with more severe brain injury; the most common locations include the petrous temporal bone, the basiocciput, the sphenoid bone, and the ethmoid bone.
Anterior skull base fractures traverse the paranasal sinuses and orbit, and they can disrupt the cribriform plate and optic canals. Clinical consequences include CSF rhinorrhea, periorbital ecchymosis (“raccoon eyes”), and olfactory or optic nerve damage. Trauma to the central skull base, usually from lateral impact, results in petrous temporal bone fractures and may be associated with CSF otorrhea, hemotympanum, mastoid ecchymosis (“Battle sign”), facial nerve palsy, vertigo, tinnitus, and conductive or sensorineural hearing loss. Fractures through the clivus can injure the sixth cranial nerve. Posterior skull base fractures can injure the dural venous sinuses, resulting in posterior fossa epidural hematoma or dural sinus thrombosis and venous infarct. If the fracture extends to the occipital condyles, it can lead to craniocervical instability.
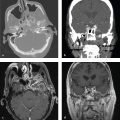
Most skull base fractures are identified on noncontrast head CT obtained for evaluation of head trauma. High-energy impact and fractures that traverse the carotid canals or cavernous sinus can be associated with carotid or vertebral injury, including dissection and pseudoaneurysm. Vascular injury places the patient at increased risk for secondary embolic cerebral infarct, which can be prevented with anticoagulation. Because of this risk, CT angiography with helical thin-section imaging is generally advised in this group ( Fig. 2.5 ).
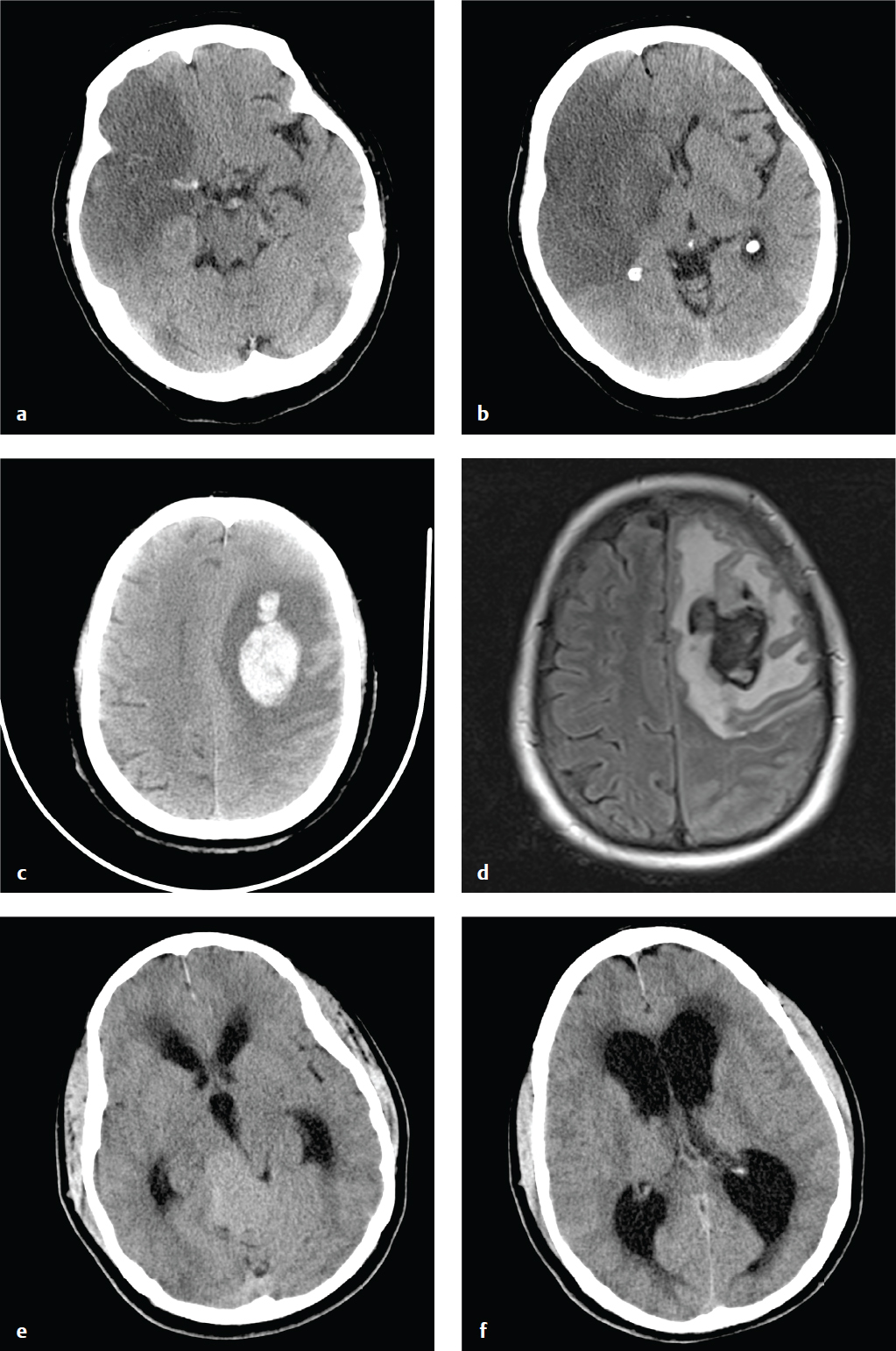
Epidural Hematoma
Most epidural hematomas (EDH) are of arterial origin and result from calvarial fractures that cross branches of the middle meningeal artery. Hemorrhage under arterial pressure separates the outer layer of the dura from the skull, creating an extraparenchymal intracranial mass that compresses the adjacent brain, leading to ischemia and potential compartmental herniation. Epidural hematomas can enlarge rapidly and, if not treated, are often rapidly fatal. With early evacuation, however, the prognosis is good; the skull absorbs most of the energy of impact as it fractures, sparing the underlying brain parenchyma from direct injury.
An acute EDH is a smooth, hyperdense biconvex extraparenchymal blood collection, limited by the coronal sutures, to which the dura is especially adherent. Hyperacute, active bleeding has an inhomogeneous, swirling appearance due to a mixture of clotted and unclotted blood.
Venous epidural hematomas, which account for approximately 10% of EDHs, are the consequence of dural venous sinus disruption or, rarely, diploic vein or arachnoid granulation rupture. They are low-pressure hemorrhages that typically do not enlarge over time and rarely require evacuation. In contrast to arterial hematomas, traumatic venous EDHs often occur in children and are not necessarily associated with skull fractures. They are most commonly seen in the middle cranial fossa adjacent to the greater wing of the sphenoid bone, where venous EDHs are due to disruption of the sphenoparietal venous sinus. They also may follow injury to the sagittal or transverse sinuses and can traverse the tentorium at the occiput or the falx at the vertex ( Fig. 2.6 ).
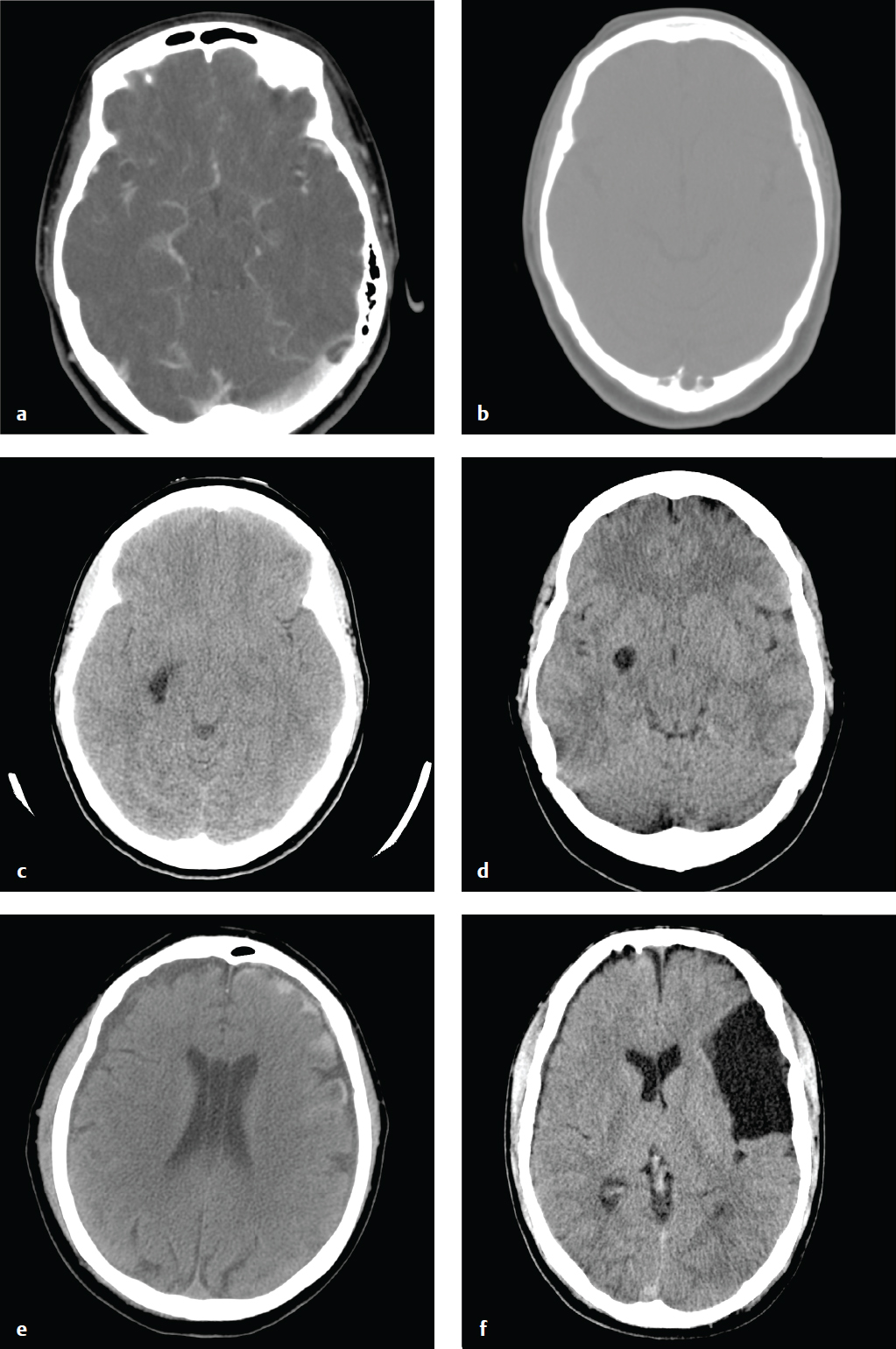
Acute Subdural Hematoma
Acute subdural hematomas (SDHs) occur when cortical veins that traverse the subdural space are torn under cerebral acceleration or rotation. Blood accumulates between the inner (meningeal) layer of the dura, which is firmly attached to the skull, and the smooth arachnoid membrane loosely adherent to the surface of the brain. Uncommonly, a primary parenchymal or subarachnoid hemorrhage can disrupt the arachnoid membrane and rupture into the subdural space. SDH is associated with a skull fracture in less than 50% of cases and is typically a “contrecoup” injury that results from recoil of the brain away from the inner surface of the skull opposite the site of impact.
Especially in younger patients, an acute SDH indicates significant energy transfer and is frequently associated with severe parenchymal brain injury, brain herniation, and cerebral ischemia. SDH due to minor trauma is more common in the elderly and in patients with cerebral atrophy. Parenchymal volume loss and enlarged CSF spaces allow the brain to move more freely in the calvarium and compromise the ability of the brain to tamponade low-pressure hemorrhages. In such patients, enlarged extracerebral space limits the degree of parenchymal compression and ischemia.
Acute SDHs are crescentic, hyperdense, and usually homogeneous. They can extend along the entire hemisphere and are confined by the falcine and tentorial dural reflections. In contrast to most EDHs, SDH is not limited by calvarial sutures. Venous hemorrhage is usually more gradual than arterial bleeding, and SDHs can develop more slowly than EDHs. Nonetheless, large SDHs can cause acute and severe neurologic deterioration and often require urgent evacuation. Hematoma thickness or subfalcine shift of 2 cm or greater is associated with a particularly poor prognosis.
The density of subdural clot can overlap with that of calvarial bone on narrow CT windows optimized for evaluation of brain parenchyma, and small subdural hematomas can be difficult to detect. To avoid this error, head CT obtained for trauma should always be viewed at both narrow and wide (subdural) window settings ( Fig. 2.7 ).
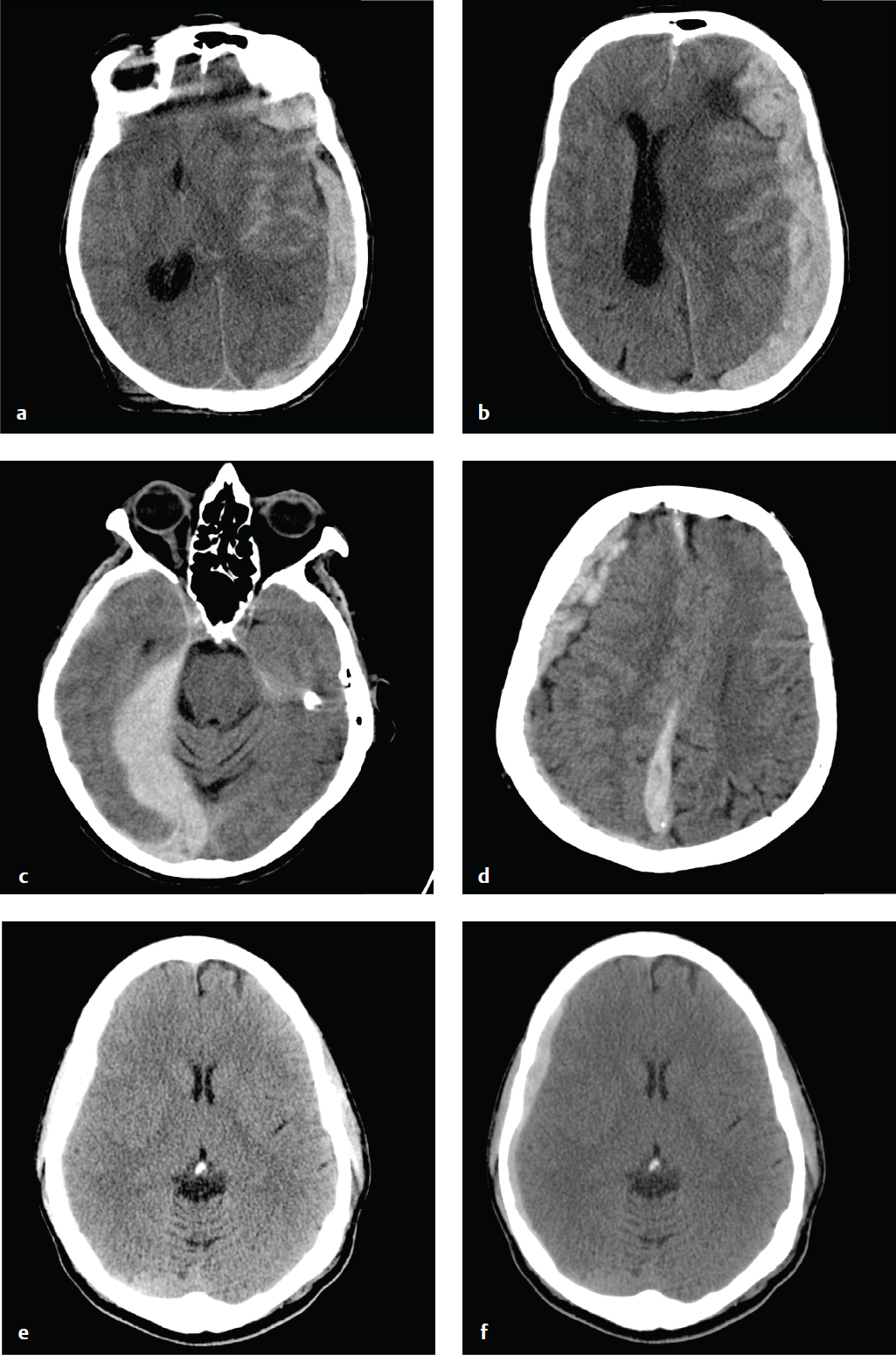
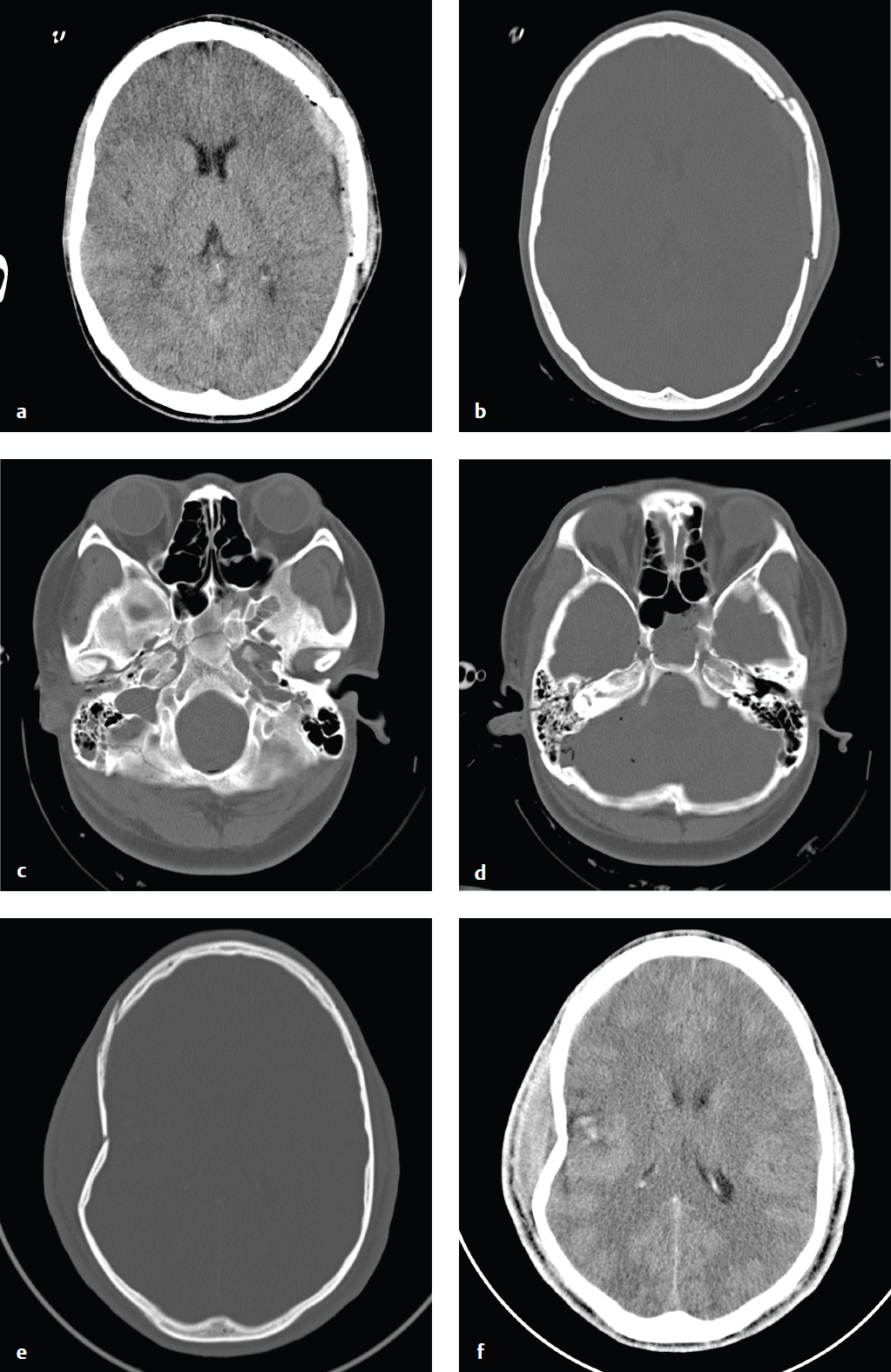
Subacute and Chronic Subdural Hematoma
SDHs evolve over time. Hyperacute hemorrhage, seen in the rare patient imaged immediately after injury, can be hypodense or mixed-density. Most patients are imaged after coagulation has taken place, and acute SDHs are typically hyperdense and crescentic. As an acute SDH ages, protein degradation occurs, extracellular fluid shifts into the hematoma, and density decreases.
SDHs that are 2 to 3 weeks old may be isodense to the adjacent cortex and difficult to detect, especially when they are small or bilateral. MRI or CT+C is more sensitive than NCCT for identification of these subtle hematomas. SDHs of any age can be complicated by rehemorrhage, which can convert a small, well-tolerated subacute SDH into a larger, symptomatic mass.
Three weeks or more after hemorrhage, a SDH is considered chronic. By this time, most simple hematomas are hypodense compared with brain and approach the density of CSF. Unevacuated hematomas incite a local inflammatory response that leads to formation of a fibrinous capsule and fragile, vascularized membranes. These can rupture spontaneously or with minor trauma, and patients who have had a previous SDH are at increased risk of hemorrhage. Delayed bleeding into the subdural space complicates up to one-third of chronic SDHs and appears as hyperdense clot within the lower-density hematoma. Rehemorrhage into a chronic SDH may also appear convex rather than crescentic due to confinement by inflammatory membranes.
Blood fluid levels can be seen in chronic or subacute SDH complicated by rehemorrhage, following lysis of the initial hematoma. Acute or subacute SDH in patients who are taking anticoagulants or are otherwise coagulopathic can also show blood fluid levels ( Fig. 2.8 ).
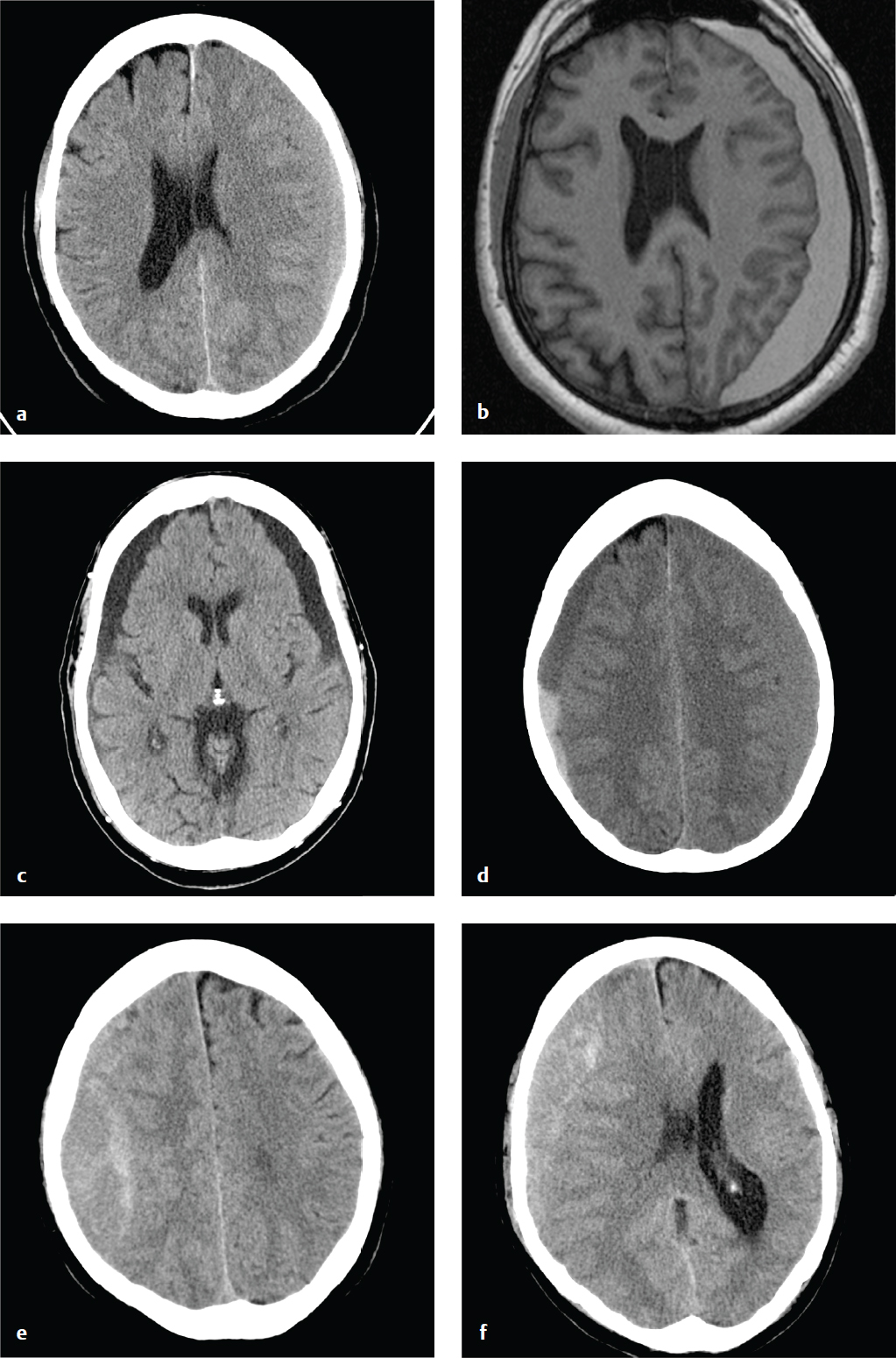
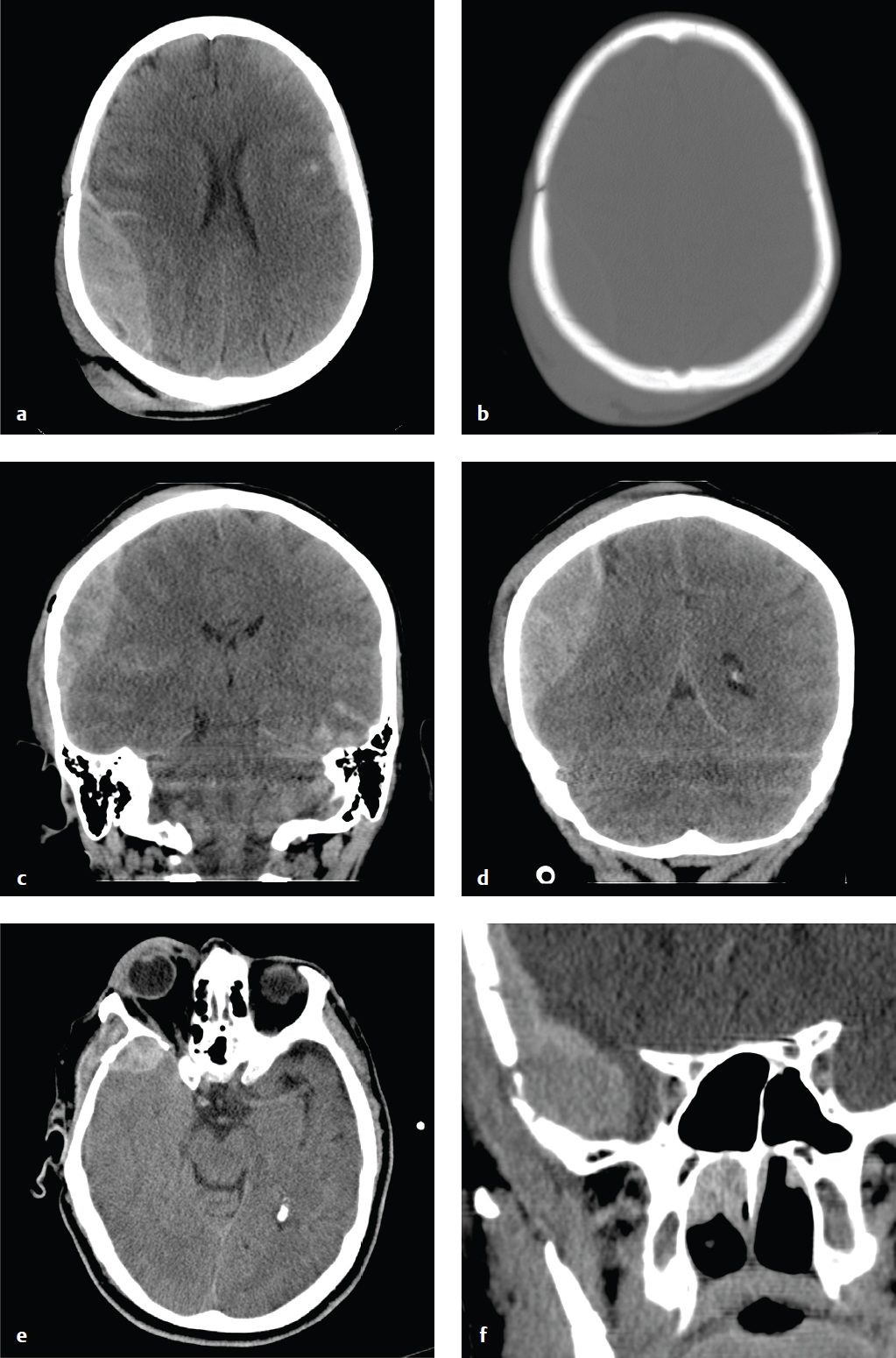
Subdural Hygroma
Subdural hygromas are due to CSF that leaks into the subdural space via either a tear or an irritation of the arachnoid. Subdural hygromas are usually bilateral, located over the anterior frontal or temporal lobes, and develop 2 to 3 days after acute head trauma.
Depending on CSF absorption and post-traumatic brain swelling, subdural hygromas can fluctuate in size over time. Atrophy or encephalomalacia affords a potential space for the development of hygromas, whereas parenchymal expansion and fluid reabsorption will speed resolution of the hygroma.
Subdural hygromas have the same CT density as CSF and can be indistinguishable from chronic subdural hematomas. When isolated and identified in the setting of acute trauma, subdural hygromas are considered a benign consequence of head injury, since most are small and clinically insignificant, and do not require surgical intervention. Subdural hygromas are commonly seen in conjunction with other brain injuries, such as contusion, traumatic subarachnoid hemorrhage, and extra-axial hematomas ( Fig. 2.9 ).
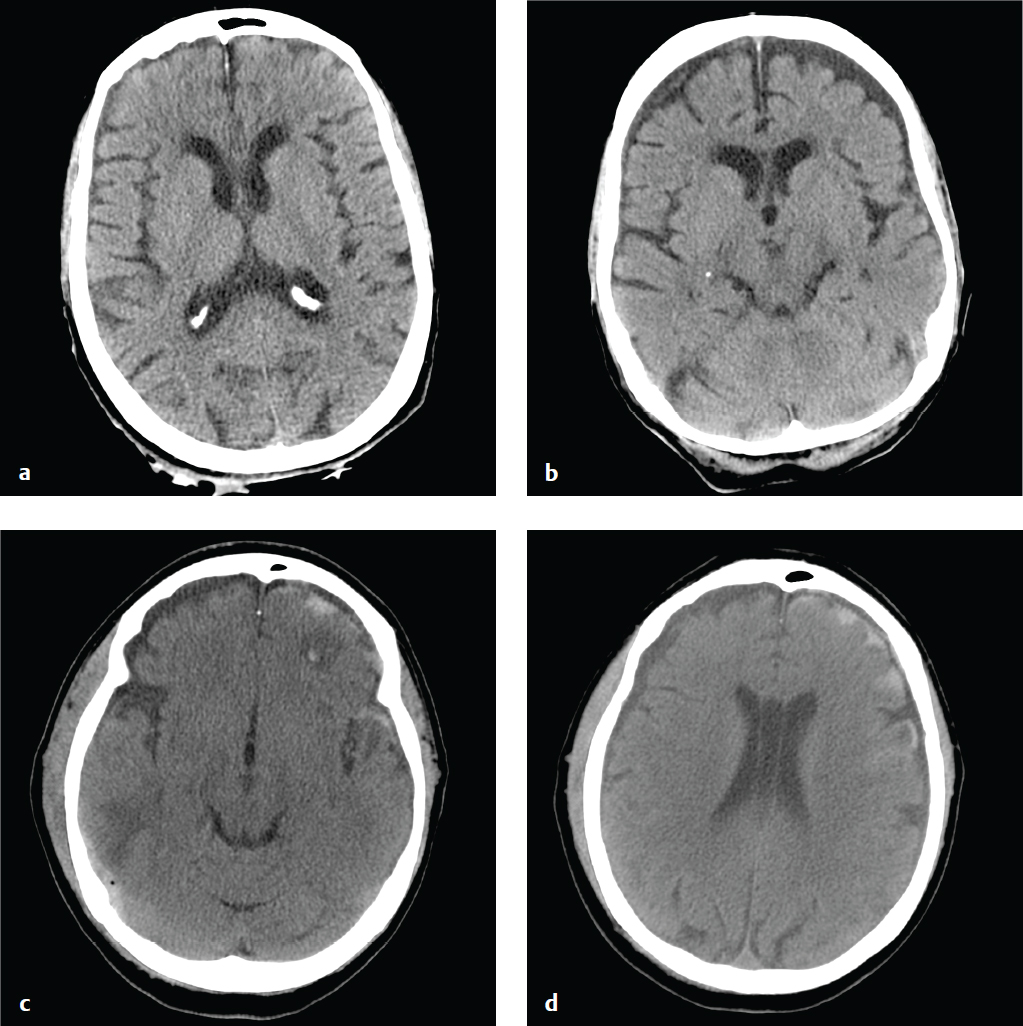
Traumatic Subarachnoid Hemorrhage
Traumatic subarachnoid hemorrhage (SAH) is a consequence of either direct pial vascular injury or extension from a hemorrhagic cortical contusion, parenchymal hematoma, or extra-axial hematoma. Although traumatic SAH is often seen in association with other brain injuries, it can be subtle, particularly if it is the only finding in minor head trauma. Blood that collects between the pia and the arachnoid membrane fills cerebral sulci and forms fine serpentine juxtacortical high-density collections. Hemorrhage is also common in the perimesencephalic cisterns. Over a period of days, blood becomes isodense to brain and ultimately resolves.
Traumatic SAH may be limited to cortical sulci, the interpeduncular cistern, or the posterior perimesencephalic cisterns. Extensive hemorrhage in the suprasellar and middle cerebral artery cisterns, especially in the absence of other traumatic brain injuries (contusion, subdural hematoma, epidural hematoma) or with minor trauma, is more consistent with subarachnoid hemorrhage due to a ruptured aneurysm. It is important to keep in mind that the accident leading to a patient′s head injury may have been precipitated by loss of consciousness due to an aneurysmal hemorrhage. CT or conventional angiography can be performed to address this possibility.
SAH is associated with increased morbidity and mortality in trauma, reflecting its potential to induce vasospasm and its association with more severe mechanisms of head injury. Subarachnoid blood is also toxic to the underlying cortex and can interfere with normal CSF absorption, which can lead to later complications of chronic hydro-cephalus or superficial siderosis ( Fig. 2.10 ).
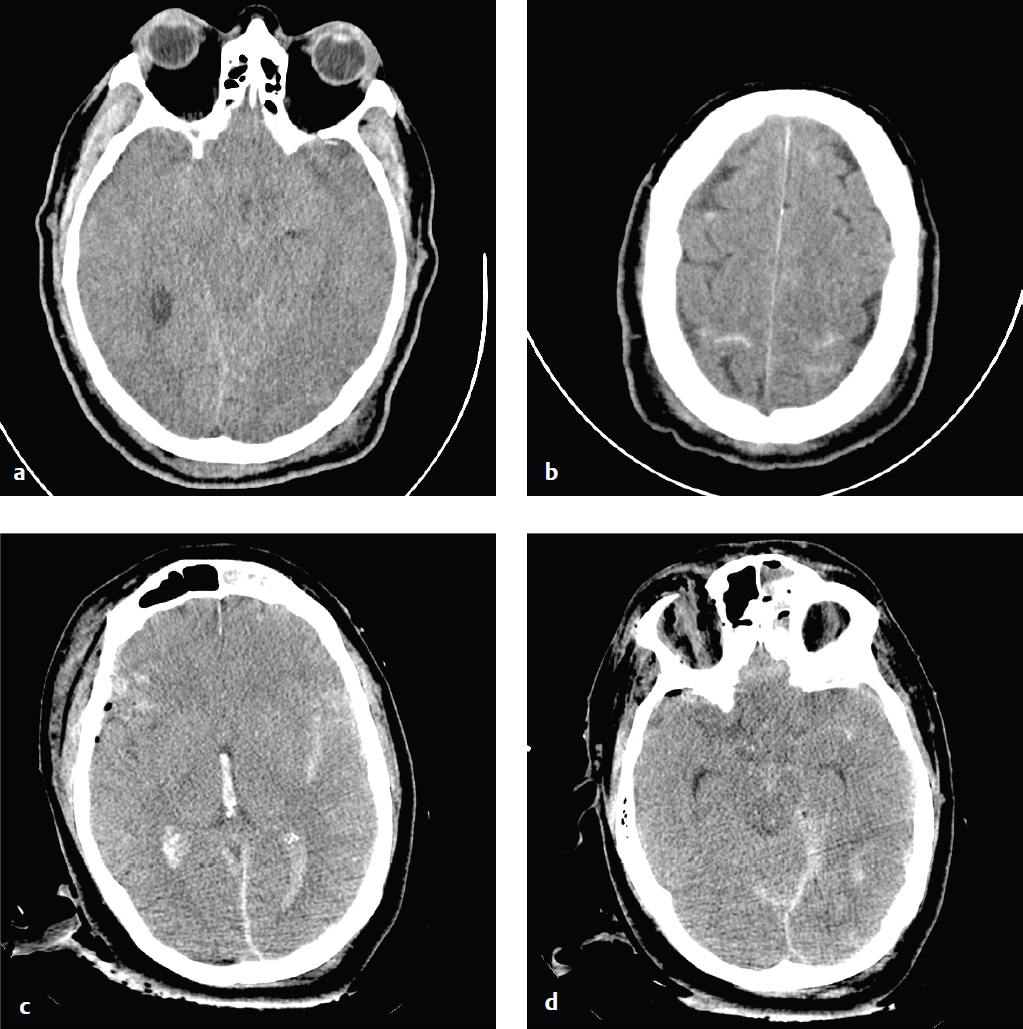
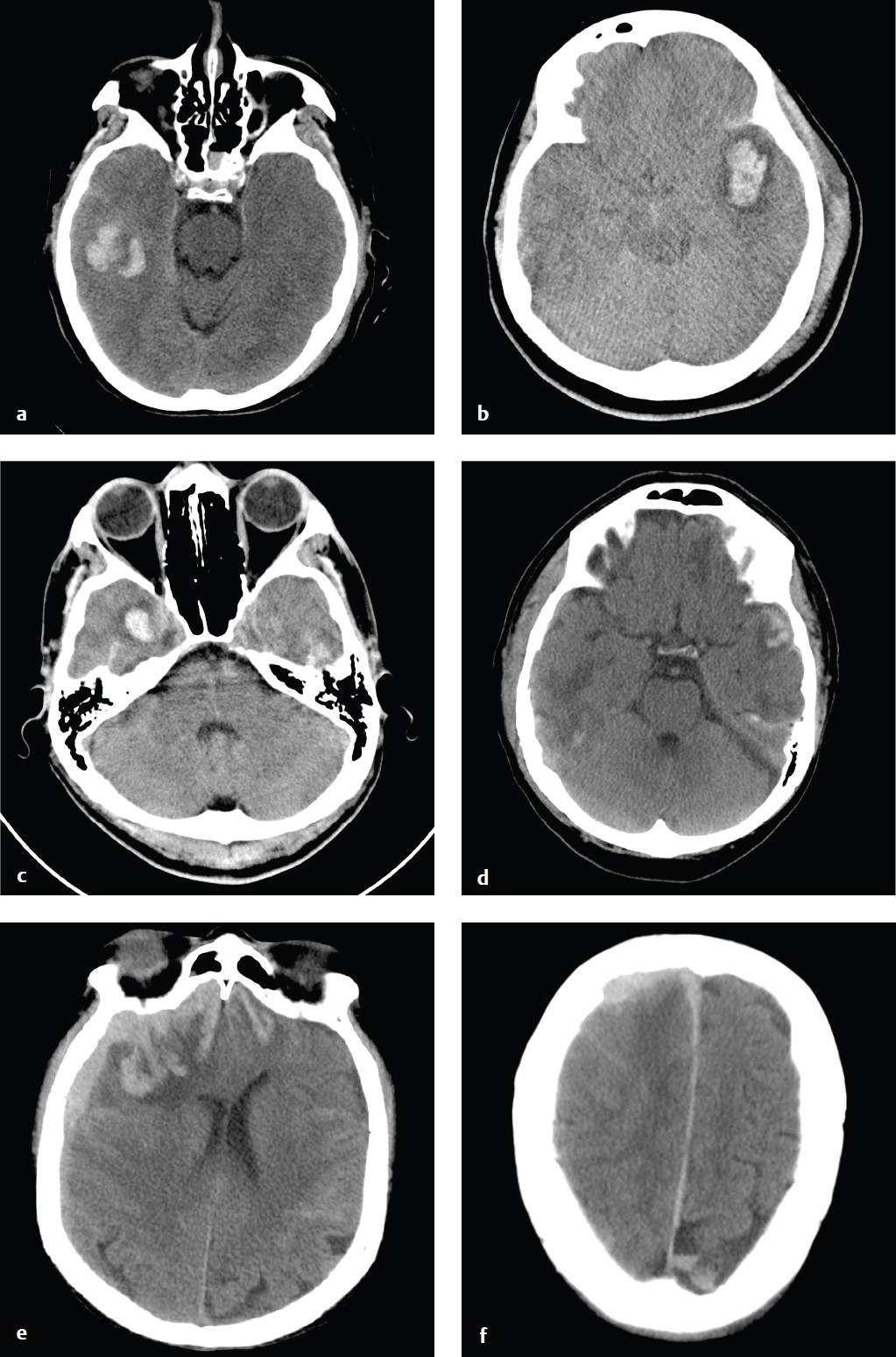
Cerebral Contusion
Cortical contusions result when the brain impacts upon the irregular contours of the orbital roofs, petrous ridges, and sphenoid wings in acute acceleration injury. As a result, most contusions are located in the cortex and immediate subcortical white matter of the inferior frontal lobes, anterior or inferior temporal lobes, posterior cerebellum, dorsal occipital lobes, and frontal and parietal convexities. They may be either coup or contrecoup in location, although the latter are more common. Contusions typically carry a better prognosis than diffuse axonal injury (DAI) unless associated with significant brain swelling.
CT is the first and often only investigation in the emergency setting; MRI is more sensitive for detection of small hemorrhages, which can be important for accurate prognosis. Contusions may be subtle or imperceptible immediately following an acute injury. Delayed hemorrhage, occurring at 12 to 48 hours, is known as posttraumatic apoplexy and reflects hypocoagulability that often develops following head trauma and resolution of acute swelling that serves to tamponade small vascular injuries ( Fig. 2.11 ).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree