2 Imaging of Gastrointestinal Hemorrhage and Acute Mesenteric Ischemia
Siva P. Raman and Elliot K. Fishman
2.1 Introduction
Multidetector computed tomography (MDCT) now serves as the first-line imaging modality in the emergency department for the diagnosis and risk stratification of a variety of bowel and mesenteric abnormalities. The rapidly increasing use of MDCT in the emergency setting and its increasing diagnostic efficacy have been driven by a variety of technological improvements over the last several generations of scanners, including equipment with better spatial and temporal resolution and the development of increasingly sophisticated study acquisition protocols. In particular, improvements in the temporal resolution of scanners have allowed the consistent, reproducible acquisition of images at peak arterial enhancement, greatly facilitating the evaluation of a variety of vascular disorders of the bowel and mesentery. MDCT images can now be acquired with beautiful delineation of the central mesenteric arterial vasculature (including small branch vessels), and these images can also be used to accurately gauge subtle sites of bleeding or abnormal bowel wall enhancement.
This chapter will discuss the role of MDCT in two of the most clinically important of these vascular disorders, namely acute gastrointestinal (GI) hemorrhage and acute mesenteric ischemia, with a focus on appropriate scan protocols, imaging diagnosis, and diagnostic pitfalls.
2.2 CT Protocol Design
When bowel pathology is suspected prospectively (such as in cases of bowel ischemia or acute GI bleeding), it is imperative that positive oral contrast (i.e., contrast agents with Hounsfield attenuation values > 50) not be used, as dense contrast within the bowel lumen not only will obscure sites of active bleeding or contrast extravasation but also will prevent accurate assessment of the bowel wall (in terms of either enhancement or thickness) as a result of beam hardening or streak artifact. In addition, in our particular practice, where a great deal of emphasis is placed on the creation of 3D reconstructions to aid in diagnosis, we are very cognizant that positive oral contrast material may also interfere with standard reconstruction algorithms. Instead, the patient should be scanned without the use of an enteric contrast agent; alternatively, a neutral agent (i.e., contrast agents with densities close to that of simple fluid) such as VoLumen or water should be used to achieve bowel distension without compromising optimal evaluation of bowel wall thickness and enhancement. Given the acuity of presentation of both of the disorders discussed in this chapter (GI hemorrhage and ischemia), it is not at all uncommon in our institution to scan patients in the emergency setting without using any oral contrast agent so as to not unduly delay the patient′s diagnosis and treatment. 1 , 2
Patients with suspected GI hemorrhage or mesenteric ischemia should be scanned using a dual-phase protocol, with the acquisition of both arterial and venous phase images after the brisk (4–5 mL/s) injection of a nonionic intravenous contrast agent (100–120 mL contrast volume). Arterial phase images are most often acquired using a bolus trigger technique (usually at approximately 30–40 s) during inspiration, whereas venous phase images are acquired using a fixed delay of approximately 50 to 60 seconds during inspiration. Images are routinely acquired using thin collimation (0.625–0.75 mm), with standard reconstruction of images into 3- to 5-mm slices for axial image review; isotropic 0.5- to 0.75-mm images are sent to an independent workstation for generation of 3D reconstructions. Commonly used 3D reconstruction techniques for the analysis of bowel and mesenteric pathology include maximum intensity projection (MIP) images, which highlight the brightest voxels in a dataset and are particularly useful for evaluation of the mesenteric vasculature, and volume-rendered images, which are 3D images created by assigning a specific color and transparency to each voxel in a dataset and are useful for providing comprehensive evaluation of the bowel. 1 , 2 Additionally, coronal and sagittal multiplanar reformations, which are critical for evaluation of the mesenteric vessel origins and for providing an overview of the entirety of the bowel, are automatically generated at the scanner for routine radiologist review. 1 , 2 , 3
Dual-phase technique is critical in cases of suspected GI hemorrhage, as having two phases allows maximum sensitivity for detecting sites of active contrast extravasation (i.e., bleeding) and differentiating between sites of active bleeding and intrinsically high-density material in the bowel (e.g., medications, old barium). Intrinsically high-density material should appear identical on the two phases, whereas a true bleed should change in both size and shape. 1 , 2 Some practices also incorporate a third noncontrast phase (with images acquired before the administration of intravenous contrast) to better differentiate between true sites of bleeding and intrinsically high-density material, although, in theory, this distinction should be possible using a biphasic acquisition. The use of dual-energy CT for GI bleeding cases also allows for the creation of virtual noncontrast images that can aid in the interpretation of these studies without the need for a separate noncontrast acquisition. 4 , 5
Dual-phase technique is also necessary in cases of mesenteric ischemia, as the two phases allow a comprehensive evaluation of the arterial and venous mesenteric vasculature for patency and offer increased sensitivity for subtle changes in bowel wall enhancement (which may be more apparent on one of the two contrast phases). 1 , 2
2.3 Acute Lower GI Bleeding
Lower GI bleeding, defined as bleeding distal to the ligament of Treitz, is a very common problem in the emergency setting, accounting for up to 3% of all inpatient admissions. Although the vast majority of bleeding episodes are self-limited, rebleeding occurs in a sizable percentage of patients (up to 20%), with patients at higher risk of morbidity and mortality in the setting of rebleeding. 6
There is no perfect radiologic modality for the evaluation of acute GI bleeding, and there has been little consensus in the literature as to the best initial option. Technetium-99m-tagged red blood cell (RBC) scans are considered quite sensitive for detecting sites of bleeding, with the ability to detect bleeding rates as low as 0.1 mL/min, and offer the advantage of potentially detecting intermittent bleeding because of their prolonged acquisition times. However, this modality suffers from poor spatial resolution and a frequent inability to accurately localize the site of bleeding, raising questions regarding its true utility in terms of guiding angiographic treatment. Moreover, tagged RBC scans do not provide information about the etiology of bleeding (e.g., diverticular disease, ischemia), also limiting its use in terms of guiding treatment. 1 , 2 Angiography is quite effective as a treatment modality but is relatively insensitive for the detection of bleeding, only detecting rates of bleeding of approximately 1 mL/min and higher. Moreover, angiography is insensitive to intermittent bleeds and is ineffective in identifying subtle bleeds because of its relatively limited contrast resolution. Traditionally, colonoscopy has been considered the first-line modality for the evaluation of acute GI bleeding in the emergency setting. However, colonoscopy can be technically challenging in the acute setting because of a lack of adequate bowel preparation; moreover, this procedure does not provide evaluation of the small bowel for bleeding sites. 2 , 6
Overall, MDCT has been shown to be quite sensitive for detecting sites of active bleeding, is able to detect rates of bleeding as low as 0.35 mL/min, and has the additional advantage of being able to provide information about the cause of bleeding (e.g., Crohn′s disease, diverticular disease, bowel ischemia). MDCT can also be used to evaluate the entirety of the bowel (both small and large bowel), unlike colonoscopy. 1 , 2 , 6 In addition, research has suggested that MDCT may be valuable for triage in the emergency department, as patients with a negative CT scan are unlikely to have subsequent episodes of rebleeding. CT may also be valuable when performed before colonoscopy to better delineate the likely site of bleeding, thus helping to guide angiographic or colonoscopy-based treatment. 7 Overall, given the rapidity of scan acquisitions using modern technology, CT imaging upon initial presentation is unlikely to unduly delay other diagnostic or treatment options, and accordingly, there is little downside to using CT before further investigation or treatment with other modalities. In general, reports in the literature have suggested relatively high sensitivities and specificities for CT in the diagnosis of acute GI bleeding, with sensitivities ranging from 79 to 100% and specificities ranging from 85 to 100%. 8 , 9 , 10 , 11 , 12 , 13
Although CT certainly has a significant, and growing, role in the diagnosis and evaluation of lower GI bleeding, there are currently no convincing data supporting the use of CT for upper GI bleeding (defined as bleeding proximal to the ligament of Treitz). In patients with symptoms clearly suggestive of upper GI bleeding (or symptoms that might possibly represent upper GI bleeding), upper endoscopy should be the primary test of choice. 6
As mentioned earlier, proper technique is of the utmost importance in studies performed for suspected GI bleeding, as poorly designed protocols will make diagnosis (particularly of active extravasation) nearly impossible. Specifically, positive oral contrast agents cannot be used for this indication, as high-density contrast agents will obscure sites of active extravasation. In general, neutral agents such as water or VoLumen can be used but are not absolutely necessary, particularly given that these studies are often performed in acutely ill patients who may not be able to tolerate drinking contrast. Although there is some argument that the ingestion of water or VoLumen may actually dilute sites of active extravasation, making their identification more difficult, we have used water as a contrast agent at our institution for several years and have not experienced any significant problems in identifying sites of bleeding. 1 , 2
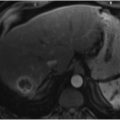
Sites of active bleeding are visible as high-density material within the bowel lumen, with attenuation identical to that of the blood pool on any given phase of contrast (▶ Fig. 2.1, ▶ Fig. 2.2, ▶ Fig. 2.3, ▶ Fig. 2.4, ▶ Fig. 2.5). The morphology of this active extravasation will depend on the rapidity of the bleed, with rapid bleeds often demonstrating a linear or “jet-like” morphology (or even resulting in a contrast level within the bowel lumen) and slower bleeds demonstrating a more ill-defined or amorphous pooling of contrast. 1 , 2 In addition, active bleeding in the bowel (particularly the small bowel) may induce hyperperistalsis, causing the actively extravasated contrast to move more distally in the bowel lumen between the arterial and venous phases. Any suspected site of bleeding must be confirmed on both the arterial and venous phase images, with a true active bleed demonstrating a change in both size and shape/morphology (▶ Fig. 2.1, ▶ Fig. 2.2, ▶ Fig. 2.4). Alternatively, the site of bleeding can be contrasted with intrinsically high-density material within the bowel lumen, which should remain identical in both size and morphology between the arterial and venous phase images. If noncontrast images are acquired, this phase can serve as a valuable means of differentiating between intrinsically high-density material and a true bleed, as intrinsically high-density material (unlike bleeding) should be seen on noncontrast images. 2 , 3 , 11 , 14 , 15 In some cases, even though frankly extravasated contrast may not be visible, a high-density clotted blot (measuring 40–70 HU) may be seen within the bowel lumen, providing a clue as to the site of bleeding (“sentinel clot” sign) (▶ Fig. 2.6). 3 Although this is not as specific as active extravasation, this sentinel clot sign may still prove valuable for helping to guide the endoscopist or interventional radiologist.






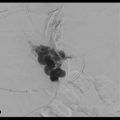
In addition to identifying sites of active extravasation, the true value of MDCT relative to other imaging modalities and colonoscopy is its ability to diagnose multiple potential causes of GI bleeding, even in the absence of active extravasation. One area of diagnosis in which MDCT particularly excels is in the diagnosis of a range of vascular abnormalities that can cause GI bleeding such as angiodysplasia, varices in patients with portal hypertension (particularly rectal varices), and arteriovenous malformations (AVMs) (▶ Fig. 2.7, ▶ Fig. 2.8). Angiodysplasia, a relatively common diagnosis in elderly patients, is often visible as an unusual tangle of vessels within the submucosal layer of the bowel (particularly the right colon), often with a dilated feeding artery and an early draining vein. 3 Bowel AVMs are a relatively uncommon finding on imaging but may represent the source of bleeding in a sizable percentage of patients with occult lower GI bleeds. Bowel AVMs are most commonly identified in the proximal small bowel but can be seen anywhere within the GI tract. 2 Finally, varices around the rectum are not uncommon in patients with portal hypertension, and although these varices are usually asymptomatic, they can sometimes cause slow, intermittent bleeding (▶ Fig. 2.8).


In addition to vascular abnormalities, MDCT also offers the advantage of a complete evaluation of the small and large bowel for a number of other inflammatory, infectious, and ischemic causes of GI bleeds, including mesenteric ischemia, inflammatory bowel disease (either ulcerative colitis or Crohn′s disease), a variety of small and large bowel tumors, Meckel′s diverticulum, and fecal impaction. 3 Although diverticular disease (in the absence of frank diverticulitis) is an incredibly common incidental finding in a large percentage of asymptomatic patients, it is a finding that should not be ignored in the setting of unexplained lower GI bleeding. In particular, diverticulosis may account for up to 40% of all cases of lower GI bleeding as well as approximately 50% of patients admitted for rebleeding. Diverticular bleeds are not uncommonly arterial in nature and can produce substantial bleeding with evidence of active extravasation on CT. If no other etiology is identified, the presence of significant diverticulosis (even without evidence of active extravasation) should be noted in the radiologist′s dictated report. 8
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree