2 Normal Anatomy
Brain Parenchyma
The major divisions of the human brain include the cerebrum, cerebellum, and brainstem. The cerebrum is further subdivided into the frontal, parietal, occipital, and temporal lobes, and the insular cortex. The frontal lobe is separated posteriorly from the parietal lobe by the central sulcus. The sylvian fissure separates the frontal lobe from the temporal lobe inferiorly. There are lateral, polar (the most anterior region), orbital, and medial parts of the frontal lobe. Sulci of note include the central sulcus, which divides the precentral gyrus anteriorly (containing primary motor cortex) from the postcentral gyrus posteriorly (containing primary somatosensory cortex), the precentral sulcus (with the superior, middle, and inferior frontal gyri lying anteriorly, and the precentral gyrus posteriorly), the superior frontal sulcus (dividing the superior and middle frontal gyri), and the inferior frontal sulcus (dividing the middle and inferior frontal gyri). Broca’s area, with functions related to speech production, lies in the dominant hemisphere (the left side, in right-handed individuals), within the pars opercularis and pars triangularis of the inferior frontal gyrus.
The parietal lobe lies behind the frontal lobe and is anterior and superior to the occipital lobe. The somatosensory cortex lies just posterior to the central sulcus within the postcentral gyrus, with the homunculus commonly used to illustrate the location therein of the different body regions (the homunculus is also used in reference to motor cortex). The sylvian fissure divides in part the parietal lobe from the temporal lobe inferiorly, with the parietal-occipital sulcus/fissure (seen medially) dividing those two respective lobes.
The occipital lobe contains the primary visual cortex, which lies medially within the calcarine sulcus. Above this sulcus is the cuneus, and below the lingual gyrus. The occipital lobe rests on the tentorium. There is no clear-cut division on the lateral surface of the brain between the occipital lobe and the neighboring parietal and temporal lobes. A theoretical dividing line can be drawn extending from the parietooccipital fissure to the temporo-occipital incisure.
Within the medial temporal lobe lies the hippocampus, which is critical for memory formation. Within the temporal lobe are also areas for auditory and visual processing of sensory input. In the dominant hemisphere, the temporal lobe contains the primary auditory cortex, with Wernicke’s area (critical for speech processing) located posteriorly in the superior temporal gyrus (Brodmann area 22).
The insular cortex (insula, or “island”) is a portion of cerebral cortex folded deep within the sylvian fissure. Overlying the insula laterally is a cortical area termed the operculum, which includes parts of the frontal, temporal, and parietal lobes. The central sulcus of the insula divides the anterior part from the posterior part of the insula. Within the anterior insula, there are anterior, middle, and posterior short gyri (which converge to the apex anteriorly), and within the posterior insula, there are anterior and posterior long gyri.
The corpus callosum (CC) is the largest interhemispheric commissure in the brain. The CC is divided into the genu (the “knee”) anteriorly, the body, and the splenium posteriorly. There is an additional small named segment, the rostrum (coming from the Latin and meaning “beak,” as on a bird), which is a continuation of the genu and projects posteriorly and inferiorly.
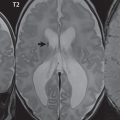
Other links between the two cerebral hemispheres include the very small anterior and posterior commissures. The line connecting these two commissures, the AC-PC line, today defines the standard axial plane for MR acquisitions. These structures cannot be identified on CT, with the orbitomeatal line (OML)—connecting the lateral canthus of the orbit and the external auditory meatus—widely used to define the axial CT plane. This leads to difficulty in clinical comparison of axial MR and CT scans of the brain, with the two lines differing on average by slightly more than 10 degrees. Critical to image interpretation, and particularly valuable for imaging followup, are appropriate standardization and consistency in acquired and displayed imaging planes and axial tilt ( Fig. 2.1 ).

The cerebellum lies in the posterior fossa, with the fourth ventricle, pons, and medulla anteriorly. Like the cerebrum, the cerebellum is divided into two hemispheres. However, in addition, there is a narrow midline zone, the vermis. The large folds of the cerebellum divide the structure into 10 smaller lobules. The primary fissure of the vermis divides that structure into anterior and posterior lobules. Readily recognizable anatomic portions of the cerebellum on imaging include the nodulus (the anterior end of the inferior vermis, which projects into the fourth ventricle inferiorly like a thumb), the flocculus (a small lobule that is lateral to the medulla and inferior/medial to the internal auditory canals), and the cerebellar tonsil (a round lobule medially on the undersurface of the cerebellum). The cerebellar cortex itself is made up of very tightly folded layers, the folia. Although there are four deep cerebellar nuclei, the dentate nucleus is by far the largest and the only nucleus consistently recognized and described clinically on MR. The cerebellum plays a major role in motor control. The cerebellar peduncles connect the cerebellum to the brainstem. The superior and inferior cerebellar peduncles are small in size, with the middle cerebellar peduncle by far the largest.
The brainstem is composed of the midbrain, pons, and medulla oblongata. Cranial nerves III through XII originate from the brainstem, with nuclei of cranial nerves V through VIII within the pons. The two main components of the pons are the ventral (anterior) pons and the dorsal tegmentum. The ventral pons consists predominantly of white matter tracts with transverse fibers. The medulla has an anterior median fissure, with a raised area on either side, the pyramids (containing the pyramidal tracts). The swellings just lateral to the pyramids contain the inferior olivary nuclei. Axons of the corticospinal tract (motor) travel through the posterior limb of the internal capsule, thence through the cerebral peduncle anteriorly and into the anterior medulla (forming the pyramid medially). Below this prominence, the majority of axons cross to the opposite side. Other major tracts include the posterior (dorsal) column—medial lemniscus pathway (PCML, sensory—mechanoreceptors and proprioceptors) and the spinothalamic tract (sensory—nociceptors and thermoreceptors). Both convey information from the body to the postcentral gyrus. Neurons of the PCML travel within the dorsal column of the spinal cord, synapse in the medulla, cross over to the contralateral side of the medulla in the medial lemniscus, and ascend to the thalamus and subsequently to the somatosensory cortex. Neurons of the spinothalamic tract, after entering through the dorsal root (like the PCML), synapse in the dorsal horn and then cross to the contralateral side of the spinal cord and ascend in the anterolateral quadrant through the brainstem to the thalamus, and from there to somatosensory cortex.
For more detail in terms of an anatomical atlas, the reader is referred to the many computer-based atlases, including The Human Brain in 1969 Pieces (Thieme, 2014).
Arterial Anatomy
Today, in the most widely used numbering system, there are seven recognized segments (C1 to C7) of the internal carotid artery (ICA): the cervical, petrous, lacerum, cavernous, clinoidal, ophthalmic, and communicating (terminal) segments. C1 (the cervical segment) extends from the carotid bifurcation to the skull base, with no branches. At its origin, the internal carotid artery is somewhat dilated, forming the carotid bulb. C2 (the petrous segment) has three sections, the ascending (vertical), genu (a 90-degree bend), and horizontal portions. Petrous ICA branches are uncommon. C3 (the lacerum segment) is short (1 cm), being that portion of the ICA passing over the foramen lacerum. C4 (the cavernous segment) is S-shaped and surrounded by the cavernous sinus, extending to the proximal dural ring. This segment is divided into the posterior vertical, posterior bend, horizontal, anterior bend, and anterior vertical portions. Prominent small branches of the cavernous ICA include the posterior trunk (the meningohypophyseal artery) and the lateral trunk. C5 (the clinoid segment) is a tiny wedgeshaped segment that extends between the proximal and distal dural rings, with this segment widest on its lateral aspect. The distal dural ring is incomplete medially, a region known as the carotid cave and a site for aneurysms. C6 (the ophthalmic segment) extends from the distal dural ring (thus being the most proximal intradural portion of the ICA) to the origin of the posterior communicating artery (PCOM). The ophthalmic artery arises anteriorly from C6, coursing laterally ( Fig. 2.2 ).

Significant branches of the ophthalmic include the central retinal and lacrimal arteries. The superior hypophyseal artery also originates from C6, typically within 5 mm of the ophthalmic origin. C7 (the communicating segment) is that segment of the artery extending from just proximal to the origin of the posterior communicating artery to the carotid terminus, where the vessel divides into the anterior and middle cerebral arteries. C6 and C7 together constitute the supraclinoid internal carotid artery. The two major branches arising from the C7 segment are the PCOM and the anterior choroidal artery. A common variant is a persistent fetal origin, in which the PCOM is prominent, equal in diameter to the P2 segment of the posterior cerebral artery, with the P1 segment usually hypoplastic. Equally common is a hypoplastic PCOM. An infundibulum, a mild (< 3 mm) symmetric dilatation at the origin of the PCOM, is considered a normal variant. The anterior choroidal artery arises posteriorly laterally from the ICA, 2 to 4 mm distal to the PCOM, and supplies the posterior limb of the internal capsule and portions of the globus pallidus, thalamus, and midbrain.
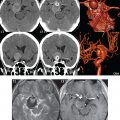
Three major arteries supply the cerebral hemispheres. The anterior cerebral artery (ACA) supplies the anterior two-thirds of the medial cerebral surface and 1 cm of superior medial brain over the convexity ( Fig. 2.3 ). Three segments are defined, A1 from the ICA to the anterior communicating artery (ACOM), A2 from the ACOM to the origins of the pericallosal and callosomarginal arteries, and A3 the subsequent distal branches. An azygous anterior cerebral artery, with a single unpaired A2 segment arising from the A1 juncture, is present in less than 1%. An accessory or duplicated anterior cerebral artery leads to the appearance of three anterior cerebral arteries (specifically three A2 segments), two laterally and one midline. This may represent persistence of a primitive median artery and is much more common than an azygous ACA. The recurrent artery of Heubner, a large lenticulostriate artery, usually originates from A2 (but may originate from A1 or the ACA-ACOM junction) and supplies the caudate head, anterior limb of the internal capsule, and the anterior part of the putamen ( Fig. 2.4 ). The posterior limb of the internal capsule and portions of the globus pallidus, thalamus, and cerebral peduncle are supplied, as previously stated, by the anterior choroidal artery, which arises from the communicating or terminal segment (C7) of the ICA. Occlusion of either of these vessels leads to a very distinctive, and not uncommon, presentation of infarction involving the basal ganglia and internal capsule.


The middle cerebral artery (MCA) supplies the lateral portion of the cerebral hemispheres, the insula, and the anterior and lateral temporal lobes ( Fig. 2.3 ). Four segments are defined, with M1 from the carotid terminus to the bifurcation (trifurcation), M2 (with superior and inferior divisions) from the bifurcation to the circular sulcus of the insula, M3 (the opercular segments) from there to the superficial aspect of the sylvian fissure, and M4 the more distal cortical branches. Note that most anatomists (as opposed to clinicians) define M1 to extend slightly post-bifurcation. An MCA bifurcation is about three times more common than a trifurcation. Branches from M1 include the lateral lenticulostriate arteries and the anterior temporal artery (supplying the anterior temporal lobe). The former arise predominantly superiorly from the M1 segment, and supply the internal capsule, caudate nucleus, putamen, and globus pallidus. The origin of the anterior temporal artery is variable.
The posterior cerebral artery (PCA) supplies the occipital lobe and the medial temporal lobe. The PCA is commonly divided into four segments, with P1 from the basilar tip to the junction with the PCOM, P2 from the PCOM to the posterior aspect of the midbrain, P3 from there to the calcarine fissure, and P4 the subsequent terminal branches. The anterior thalamoperforators arise from the PCOM, and the posterior thalamoperforators arise from the P1 segment. The latter supply parts of the thalamus, brainstem, and posterior internal capsule. The thalamogeniculate arteries (also perforators) arise from the midportion of P2 and supply the posterior lateral thalamus, posterior limb of the internal capsule, and optic tracts.
The vertebral artery has four segments, V1 from its origin (along the posterior superior wall of the subclavian artery) to the foramen transversarium of C6, V2 from there to the foramen transversarium of C1, V3 from there to the dura, and V4 the distal intradural portion. Anomalous origins from the aortic arch occur.
Three major vessels supply the cerebellum. The posterior inferior cerebellar artery (PICA), the largest of these, originates from the V4 segment of the vertebral artery about 1.5 cm proximal to the vertebrobasilar junction. PICA supplies the lower medulla, cerebellar tonsils, inferior vermis, and posterior inferior cerebellar hemisphere (the inferior cerebellum with the exception of its most anterior extent). PICA can be hypoplastic or absent (the latter in up to a quarter of cases). The vertebral artery may also terminate in PICA. The anterior spinal artery arises (bilaterally) from the vertebral artery distal to PICA and proximal to the vertebrobasilar junction.
The two vertebral arteries join to form the basilar artery near the level of the pontomedullary junction ( Fig. 2.5 ). The basilar artery terminates near the pontomesencephalic junction. The anterior inferior cerebellar artery (AICA) arises from the basilar artery approximately 1 cm distal to the vertebrobasilar junction. AICA is the smallest of the three cerebellar arteries and supplies the anterior inferior portion of the cerebellum. It is commonly stated that the distribution of AICA is in equilibrium with PICA, with at times the distribution slightly larger or smaller. The superior cerebellar artery (SCA) arises from the basilar artery immediately prior to its termination. It supplies the superior half of the cerebellum. The SCA is not uncommonly duplicated. Extensive anastomoses exist between PICA, AICA, and the SCA. Basilar artery perforators arise throughout the length of the vessel and supply the brainstem. Medial perforators enter the pons near the midline, circumflex perforators travel a variable distance around the brainstem before entering. Fenestration of the basilar artery occurs in about 1%, with fenestrations of most of the proximal segments of the major arteries supplying the brain known (for example, the A1 and P1 segments), but less common.

The circle of Willis, the ring of connecting vessels providing important collateral circulation between the internal carotid arteries and the vertebrobasilar system, is well developed and symmetric in less than half of normal individuals. Asymmetry contributes to flow patterns that are important in the development of aneurysms and ischemic stroke. In half of normal individuals, at least one component is hypoplastic. The most common variant is a hypoplastic PCOM (about onethird of patients). A fetal origin PCA is seen in about 1 in 5 patients, typically with an accompanying hypoplastic P1 segment. A hypoplastic A1 is seen in about 1 in 10 patients. The ACOM, which connects the two anterior cerebral arteries, is hypoplastic in 5 to 15%.
The external carotid artery (ECA) is the smaller of the two terminal branches of the common carotid artery. It arises anterior and medial to the internal carotid artery, then courses posterior laterally. There are many muscular branches, with the early branching of the external carotid artery allowing rapid recognition of this vessel in distinction to the internal carotid artery. There are eight major branches, which can be grouped on the basis of their ventral or dorsal origin from the ECA. The ventral external carotid branches, in order by point of origin from proximal to distal, are the superior thyroid, lingual, facial, and internal maxillary arteries. The dorsal external carotid branches, similarly ordered, are the ascending pharyngeal, occipital, posterior auricular, and superficial temporal arteries. The middle meningeal artery arises from the internal maxillary artery.
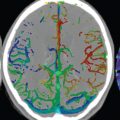
There are numerous anastomoses between ECA branches and branches of the internal carotid and vertebral arteries, with three major anastomotic pathways described. In the orbital region, the internal maxillary and internal carotid circulations interface via the ophthalmic artery. Major interfaces also exist in the petrous-cavernous region and the upper cervical region, with the latter extending between the ascending pharyngeal or occipital artery and the vertebral artery. Pialleptomeningeal anastomoses are very common and are an important potential source of collateral blood flow in occlusive vascular disease ( Fig. 2.6 ).

There are also multiple internal carotidvertebral/basilar artery anastomoses, which represent persistent embryonic circulatory patterns. One of these is seen not infrequently, as a normal variant, and is the persistent trigeminal artery. This vessel, when present, joins the proximal intracavernous segment of the internal carotid artery with the mid- or distal portion of the basilar artery. Presence of a persistent trigeminal artery is frequently associated with hypoplasia of the midsection of the basilar artery. A persistent hypoglossal artery is the second most common carotidbasilar anastomosis, arising from the internal carotid artery between the bifurcation and C1, and traversing the hypoglossal canal.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree