2 Response Assessment in Neuro-oncology
2.1 Introduction
The gold standard for outcome in oncology and in the treatment of brain tumors is measured as overall survival. Beyond showing improved survival, clinical trials have relied on surrogate markers such as progression-free survival (PFS) and radiographic response to show improvements with brain tumor treatment. As with other solid tumors, radiographic response has been the most important end point in clinical trials for brain tumors. Reduction in tumor mass has traditionally been seen as an important correlate marker for improved quality of life, longer survival, and overall clinical benefit. 1 The assessment of tumor burden in high-grade glioma (HGG) patients in clinical trials has been based on the contrast extravasation as measured on computed tomography (CT) or, more recently, by magnetic resonance imaging (MRI). This two-dimensional planar measurement has worked reasonably well in the era of cytotoxic chemotherapy, but the development of antiangiogenic agents such as bevacizumab in brain tumors has challenged this paradigm and has triggered the requirement for and development of new imaging end points. In 2010, new response criteria from the Response Assessment in Neuro-Oncology (RANO) Working Group addressed some of these challenges. 2
2.2 Traditional Assessments of Brain Tumors
Early attempts to use and standardize imaging criteria for evaluating patients undergoing therapy for HGGs go back to the 1970s when CT scans were first used to define tumor progression in brain tumor patients. 3 In 1990, the so-called Macdonald criteria further developed the Levin criteria and established, for the first time, a set of objective radiological and clinical response criteria for the standardized assessment of brain tumor response. 4 They provided a standardized radiological assessment tool for tumor response in clinical trials and were based on the two-dimensional measurement of tumor enhancement with contrast (sum of the products of the maximum perpendicular tumor diameters) (Table 2.1). In addition to the imaging features, the so-called Macdonald criteria also considered the impact of corticosteroids and changes in neurologic status of patients. This new set of criteria enabled response rates to be standardized for clinical trials and has been widely used in clinical trials. Although the Macdonald criteria were originally developed for use in CT scans, they were successfully extrapolated to MRI as well. In either case imaging interpretation is based on the disruption of the blood–brain barrier and the extravasation of contrast material into the peritumoral brain tissue.
Response | Definition |
Complete response | Requires all of the following: |
Complete disappearance of all enhancing measurable and nonmeasurable disease sustained for at least 4 weeks | |
No new lesions | |
No corticosteroids | |
Clinically stable or improved | |
Partial response | Requires all of the following: |
50% or more decrease compared with baseline in the sum of products of perpendicular diameters of all measurable enhancing lesions sustained for at least 4 weeks | |
No new lesions | |
Stable or reduced corticosteroid dose | |
Clinically stable or improved | |
Stable disease | Requires all of the following: |
Does not qualify for complete or partial response, or progression | |
Clinically stable | |
Progression | Defined by any of the following: |
25% or more increase in sum of the products of perpendicular diameters of enhancing lesions | |
Any new lesion | |
Clinical deterioration | |
Source: Data from Macdonald DR, Cascino TL, Schold SC Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol 1990;8(7):1277–1280. | |
During the same time period, one-dimensional assessment of solid tumors became the standard to assess response to treatment. The Response Evaluation Criteria in Solid Tumors (RECIST) were first published in 2000 5 and later revised in 2009. 6 The one-dimensional RECIST have been widely used in systemic cancers. Although they were found to correlate with two-dimensional and volumetric assessment in retrospective HGG studies, 7 , 8 they were inferior to three-dimensional assessment. To this point the RECIST have not been prospectively validated in brain tumors and the Macdonald criteria remain the most extensively used brain tumor imaging criteria.
2.3 Limitations of Macdonald Criteria
Although there are several limitations to using the Macdonald criteria (reviewed in detail by van den Bent et al 9 ) the most prominent is its reliance on contrast-enhancing lesions to define tumor progression. 9 Contrast enhancement in the brain is a reflection of the blood–brain barrier and is nonspecific. Although this is commonly seen in HGGs, it can also be caused by other nontumor processes such as postsurgical changes, 10 ischemia, 11 seizure activity, 12 radiation-induced pseudoprogression, or radiation necrosis. 13 The often irregularly shaped tumors make proper measurement difficult and create another layer of complexity while increasing the chances of interobserver variability. Enhancement itself can be influenced by steroids and antiangiogenic agents. 14 The use of the latter, creating the picture of a so-called pseudoresponse, triggered the requirement for the development of new imaging criteria.
2.4 Pseudoprogression and Radiation Effects
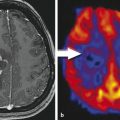
The standard of care for patients with glioblastoma (GBM) consists of maximal safe tumor resection followed by radiation with concurrent and adjuvant chemotherapy with temozolomide (Fig. 2.1). 15 On the first MRI scan following radiation therapy up to one third of patients present with contrast enhancement, which eventually subsides without changing the therapeutic regimen. 16 , 17 These occurrences are thought to be caused by a transient increase of blood vessel permeability due to radiation and are commonly called pseudoprogression. Temozolomide acts as a radiosensitizer when given concurrently with radiation therapy for GBM. 18 The incidence of pseudoprogression appears to be increased with its use during radiation and correlates with the methylation of the MGMT gene promoter. 19 This treatment effect has not been formally addressed in the Macdonald criteria while having implications on patient management and treatment evaluation. Despite the development of new radiographic assessment, such as perfusion imaging, 20 , 21 there is no reliable imaging to differentiate between true tumor progression and pseudoprogression (Fig. 2.2). In addition, the combination of radiation with chemotherapy appears to result in more frequent and earlier radiation necrosis than treatment with radiation alone. 22 The inability to differentiate between pseudoprogression, radiation necrosis, and tumor progression limits the validity of PFS as the primary end point in clinical trials. Patients with pseudoprogression who participate in clinical trials for recurrent disease would create falsely high response rates and prolonged survival rates. Although most clinical trials use a 90-day minimum interval after radiation therapy to participate in clinical trials, the Macdonald criteria have not formally addressed this issue.


2.5 Enhancement from Local Treatment
Increased enhancement can be seen postsurgically and after the administration of locally administered therapies. Because postsurgical enhancement is often developing in the wall of the surgical cavity 48 to 72 hours after resection, postoperative MRI should be performed within the first 24 to 48 hours for comparison purposes. 10 This MRI scan should include diffusion-weighted images to assess for postsurgical ischemia. Local therapies such as chemotherapy wafers, immunotherapy, and locally administered gene and viral therapies further complicate the reliance on enhancement only to diagnose tumor progression. 9 , 23
2.6 Pseudoresponse with Antiangiogenic Agents
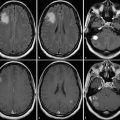
Antiangiogenic agents targeting the vascular endothelial growth factor (VEGF) and its receptor are known to produce dramatic reduction in enhancement (Fig. 2.3). The application of bevacizumab as well as cediranib can produce reduction in swelling and enhancement within 1 or 2 days of starting therapy, and radiological response rates of 25 to 60% have been described in the literature and can be observed in daily clinical practice. 24 , 25 , 26 This rapid presumed “response” to therapy is thought to be caused by a stabilization of the blood–brain barrier, based on the specific inhibition of VEGF (previously also known as vascular permeability factor). Based on the original Macdonald criteria, antiangiogenic therapy resulted in outstanding response rates, which, so far, have not been associated with major improvement in overall survival. 24 , 27

2.7 Nonenhancing Tumors
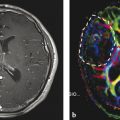
Another limitation of the Macdonald criteria is due to the infiltrative nature of HGG that does not always result in a breakdown of the blood–brain barrier and enhancement with gadolinium. 28 The Macdonald criteria do not account for the extent of nonenhancing tumor mass that is routinely observed as hyperintensity on fluid-attenuated inversion recovery (FLAIR) and T2-weighted imaging. Although being highly sensitive to new changes in the brain, FLAIR changes can be difficult to interpret because they are caused not only by tumor progression but also by peritumoral edema and radiation-induced white matter changes. The application of the Macdonald criteria has been limited in trials for low-grade (World Health Organization [WHO] II) and anaplastic tumors (WHO III), which often present without any enhancement and intact blood–brain barrier. Several studies have shown that a subpopulation of patients that showed initial response to antiangiogenic therapy subsequently presented with increasing areas of nonenhancing infiltrating disease observed on FLAIR sequences (Fig. 2.4). It has been shown that VEGF-targeting agents lead to a decrease of the vascular supply and a reduction of large- and medium-sized blood vessels. At the same time the brain tumor showed increased infiltration into the parenchyma along with increased FLAIR hyperintensity. This phenomenon is thought to be a reflection of the tumor cells’ increased usage of existing blood vessels, resulting in more tumor cell invasion and nonenhancing disease on FLAIR sequence. 29 , 30

2.8 Development of Updated Response Assessment Criteria in Neuro-oncology
The aforementioned limitations of the Macdonald criteria have led to an international effort in the neuro-oncology community to develop more specific criteria to define response in clinical trials with HGG patients. This international group effort was initiated after it became obvious that the Macdonald criteria were insufficient to assess brain tumors with the validity, objectivity, reproducibility, and comprehensiveness that are required by regulatory agencies. The RANO Working Group consisted of international opinion leaders in the field of neuro-oncology and included neuro-oncologists, neurosurgeons, radiation oncologists, neuroradiologists, and neuropsychologists as well as specialists in the assessment of quality of life. 2 The RANO Working Group includes members with leadership responsibility in major research cooperative groups in the United States and Europe. The members were selected as part of an informal process, and input of patient representative groups is not described. The suggested guidelines on how to assess response in HGG and in low-grade glioma, and the evaluation of surgically based therapies, were published in 2010, 2011, and 2012, respectively. 2 , 23 , 31 Rather than being definite, the recommendations of the RANO group should be seen as a work in progress to better define response to therapy in patients with malignant brain tumors not only in the setting of clinical trials but in daily practice as well.
2.9 The RANO Criteria
The updated response criteria for HGG by the RANO group is a further development of the Macdonald criteria and is similarly based on the serial assessment of specific lesions; however, in addition to the enhancing lesions, nonenhancing parts of the tumor are also assessed (Table 2.2). As before, the product of the maximal cross-section diameters is used to measure the contrast-enhancing lesions. 2 Similar to the Macdonald criteria, measurable enhancing lesions must have at least two perpendicular diameters that measure at least 10 mm each and that are visible on two or more slices of an MRI that are at most 5 mm apart. Cystic parts and the surgical cavity are not included in the measurement. Nonmeasurable enhancing disease is defined by lesions that are only unidimensionally measurable (i.e., masses without clear margins or with a perpendicular diameter < 10 mm). Patients with a gross total resection can achieve only stable disease as the best radiographic outcome, and patients without measurable disease cannot participate in studies intending to measure response rate as the primary outcome. However, patients without measurable disease can participate when the primary end point is duration until disease progression (tumor control).
Response | Definition |
Complete response (CR) | Requires all of the following: |
Complete disappearance of all enhancing measurable and nonmeasurable disease for at least 4 weeks | |
No new enhancing lesions | |
Stable or improved nonenhancing (T2/FLAIR) lesions | |
Off corticosteroids | |
Clinically stable or improved | |
Patients with nonmeasurable disease only, cannot have CR: best response possible is SD | |
Partial response (PR) | Requires all of the following: |
50% or more decrease compared with baseline of all measurable enhancing lesions sustained for at least 4 weeks | |
No progression of nonmeasurable disease | |
No new enhancing lesions | |
Stable or improved nonenhancing (T2/FLAIR) lesions | |
Same or lower dose of corticosteroids compared with baseline scan | |
Clinically stable or improved | |
Stable disease (SD) | Requires all of the following: |
Does not qualify for CR, PR, PD | |
No new lesions | |
Stable nonenhancing (T2/FLAIR) lesions | |
Same or lower dose of corticosteroids compared with baseline scan | |
Clinically stable or improved | |
Progressive disease (PD) | Defined by any of the following: |
25% or more increase in enhancing lesions compared with the smallest tumor measurement obtained either at baseline (if no decrease) or best response | |
Significant increase in T2/FLAIR nonenhancing lesion compared with baseline scan or best response after initiation of therapy | |
Any new lesion | |
Above imaging changes on stable or increasing doses of corticosteroids | |
Clear clinical deterioration not attributable to other causes than tumor or changes in corticosteroid dose | |
Failure to return for evaluation as a result of death, deteriorating condition, or clear progression of nonmeasurable disease | |
Abbreviations: FLAIR, fluid-attenuated inversion recovery. Source: Adapted from Wen P, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: Response Assessment in Neuro-Oncology Working Group. J Clin Oncol 2010;28(11):1963–1972. | |
2.9.1 Assessment of Multiple Lesions
In the setting of more than one enhancing lesion, a minimum of the two largest and a maximum of up to five lesions should be measured. Similarly to the RECIST, the sum of the product of the perpendicular diameters of these lesions should be calculated. 6 The RANO Working Group realizes that sometimes a smaller lesion should be favored over the largest one if it can be measured more precisely. When assessing patients with multiple lesions of which not all are increasing in size, the enlarging lesions should be the ones considered for response evaluation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree