20 Pituitary Incidentaloma and Incidental Silent Macroadenoma
20.1 Pituitary Incidentaloma
20.1.1 Introduction
A pituitary incidentaloma (PI) refers to a small, potential mass within the gland detected by an imaging study that was performed for reasons, a priori, unrelated to pituitary dysfunction or suspected dysfunction. Constant improvement in the sensitivity of MR has outpaced specificity, which may result in a diagnostic dilemma for the primary care physician.
In this chapter, we will first present a classic case of a PI that illustrates the diagnostic imaging and clinical evaluation. Important guidelines as to which tests are needed and appropriate imaging follow-up intervals will be given as well as imaging protocols for optimal evaluation of the sella.
An interesting companion case is shown to demonstrate a common incidental cystic lesion of the pituitary gland, Rathke′s cleft cyst.
A final companion case is shown to demonstrate that endocrine dysfunction is not always readily apparent and some conditions, such as acromegaly, may present insidiously and, in most cases, require surgical management. Therefore, when an incidental pituitary lesion is discovered, a detailed history and physical examination is paramount as is clinical laboratory testing.
20.1.2 Case Presentation
History
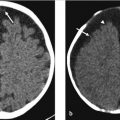
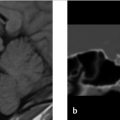
A 36-year-old woman presents with a history of left retro-orbital pain, nausea, and vomiting. Routine MRI revealed a prominent pituitary gland with a superiorly convex margin. Therefore, a dedicated pituitary MR was performed. The images are shown in ▶ Fig. 20.1.
20.1.3 Imaging Analysis
Imaging Findings
There is a small area of heterogeneous signal intensity in the left side of the pituitary gland (see arrows ▶ Fig. 20.2a-c) measuring 4.6 × 3.76 × 4.84 mm, which was not well seen on the precontrast T1-weighted (T1W) images (not shown) but is well defined postcontrast as it enhances to a lesser degree than the remainder of the gland (▶ Fig. 20.2a). The gland is slightly prominent with a superiorly convex margin, measuring 9 mm in maximum height. The cavernous sinuses are symmetric, the gland does not contact the optic chiasm, and the pituitary stalk is midline.

Imaging Impression
Probable pituitary microadenoma.
Recommendation
Clinical evaluation is recommended to determine if this is a functioning or nonfunctioning microadenoma. No additional imaging is needed. When evaluating a possible PI found on a routine brain MRI in a patient with no evidence of endocrinological dysfunction, dynamic contrast-enhanced (DCE) pituitary MRI may increase the incidence of false-positive results.
20.1.4 Clinical Evaluation
Endocrinology Evaluation
This is a 36-year-old woman with an incidentally discovered pituitary mass. She denies galactorrhea or breast tenderness. Menses are regular. There has been no change in the size of shoes, rings, or gloves, change in voice, sleep apnea, or carpal tunnel–like symptoms. There is no new sleep disturbance, excessive skin fragility, difficulty climbing stairs or rising out of the chair, lower extremity swelling, weight gain, or history of kidney stones. There is no history of diabetes, hypertension, or cardiovascular disease.
Physical Examination
Physical examination was unremarkable.
Additional Testing Results
Prolactin: 16.2 ng/mL (3–30 ng/mL).
Insulin-like growth factor 1 (IGF-1): 178 ng/mL (126–291 ng/mL).
Clinical Impression
No evidence of prolactinoma or growth hormone (GH) secreting tumor in this normal-appearing 36-year-old woman with a small pituitary mass. Most small (< 6 mm) incidentally detected lesions in the pituitary gland are nonfunctioning microadenomas or cysts. 1 At a minimum, a prolactin (PRL) and IGF-1 level are recommended. If normal, no additional workup is necessary.
Neurosurgery and Endocrinology Recommendation
The overwhelming majority of incidental microadenomas are nonfunctioning pituitary adenomas (NFPAs) that will either remain stable or decrease in size. A follow-up MR of the pituitary gland should be obtained in 1 year. Unless there is a clinical change, no follow-up laboratory testing is necessary. The recommendations made above for laboratory testing and follow-up imaging are based on current Endocrine Society and American Association of Neurological Surgeons (AANS)/Congress of Neurological Surgeons (CNS) guidelines. 2 , 3 Guidelines vary between publishing society (see ▶ Table 20.1).
20.1.5 Imaging Differential Diagnosis of Intrasellar Lesions
Common solid lesions:
Pituitary micro-/macroadenoma:
The most common solid lesion of the pituitary gland in adults, but rare in children.
Usually enhances less than the normal gland but may become hyperintense on delayed postcontrast imaging.
Pituitary hyperplasia:
Homogeneous enhancing, enlarged gland most often associated with normal physiologic hypertrophy.
Common cystic lesions:
Rathke’s cleft cyst (RCC):
Midline, nonenhancing mass, between the anterior and posterior lobes of the pituitary gland.
Often hyperintense on T1 precontrast images.
Small nodule within a cyst is considered characteristic but not always seen.
Pituitary adenoma with cystic/necrotic change:
Usually off-midline with septations or fluid–fluid levels.
Empty sella:
Pituitary infundibulum seen traversing the “empty” sella without mass effect.
Less common lesions:
Craniopharyngioma:
Nodular enhancement and calcification in a sellar/suprasellar mass are highly suggestive of this lesion. Presentation as a purely intrasellar mass is rare.
Aneurysm:
A mass that is dark on T1 W and T2 W images and in continuity with the cavernous carotid artery should raise concern and MR or CT angiography performed.
Meningioma:
Isointense to the brain on T1 W and T2 W images with homogeneous enhancement. Usually centered outside the sella. Pure, intrasellar presentation is rare.
Metastatic disease:
Rapidly enlarging mass, frequently associated with destruction of the bony sella.
Granulomatous disease:
Pituitary stalk is enlarged and abnormally enhancing.
Posterior lobe bright spot may be absent.
20.1.6 Diagnostic Imaging Pearls
Attempt to identify the “lesion” in both sagittal and coronal planes on a dedicated high-field MR examination to increase confidence that the lesion is not artifactual.
Although a small, solid mass in the pituitary gland most likely represents a pituitary adenoma (PA), clinical correlation is extremely important as microcysts, asymptomatic hemorrhage, infarcts, and nonfunctioning microadenomas are common. 7
Deviation of the pituitary stalk away from the “lesion” may be helpful but not definitive as “tilting” of the stalk is reported as a normal variant. 8
Even small, 4- to 6-mm, microadenomas will usually distort the contour of the gland, creating an upper surface bulge or depression of the sellar floor. 7
Contrast may not be necessary. Most adenomas, especially the common prolactinoma, are most frequently hypointense on non-contrast T1 W images and hyperintense on T2 W images relative to the normal pituitary gland.
20.1.7 Diagnostic Imaging Pitfalls: Artifacts and“Overcalls”
Computed tomography pitfalls:
In addition to radiation exposure, a major drawback to CT is the creation of artifactual areas of low density within the gland due to beam hardening creating streak artifact, which has been reported in 65% of CT scans of the sella, especially on the reformatted images. 9 , 10 , 11
Magnetic resonance pitfalls:
Susceptibility artifact: Prominently aerated sinus adjacent to the sella or metal within the patient’s mouth may create local magnetic field inhomogeneity that causes areas of falsely decreased signal within the adjacent skull base and sella, generating both false-positive and false-negative examinations. 12 This phenomenon is worse at higher field strength and on gradient recall echo (GRE) type images but less noticeable on fast spin echo (FSE) T2 W images.
Motion artifact: Pulsatility from cavernous carotid and patient motion artifact may create ghosting throughout the gland.
Volume averaging: Routine brain MRIs, which often employ 5-mm-thick sagittal T1W images, may create the false impression of a lesion within the pituitary gland by volume averaging pituitary tissue with the adjacent cavernous carotid artery or bony sella. Volume averaging occurs when different anatomic structures are present within the same voxel. The dedicated pituitary MR protocol uses thinner imaging cuts and allows confirmation of a lesion in two planes.
False-positive examinations for very small lesions: Diagnosing small lesions as adenomas within the gland that may be areas of normal heterogeneity is well reported. 13 Chong et al found small microlesions in the pituitaries of 40% of normal volunteers on 3-mm noncontrast T1 W images (well above the average 10–14% incidence reported in autopsy series 14 ), leading them to state that a “hypodensity in the pituitary gland was recognized as consistent with the diagnosis of a pituitary microadenoma but not in itself diagnostic of such.” 15 Microlesions may also be caused by cysts, infarcts, metastases, voxel-to-voxel heterogeneity in normal tissue, and MR system noise. 15 Teramoto et al calculated that there was a 6.1% false-positive rate when the “diagnosis of pituitary microadenoma” was made on imaging alone. 16
Is the lesion possibly vascular? Small cavernous carotid aneurysm projecting medially into the region of the sella could appear as a “lesion” that is very dark on the T1 W and T2 W images. Close inspection of the continuity of the flow void with the cavernous carotid on the coronal high-resolution T2 W images is helpful. Circle of Willis MRA or CTA is important to obtain if there is suspicion the lesion could be vascular in nature.
20.1.8 Additional Information Regarding Pituitary Lesion Imaging
Having dedicated imaging protocols for the evaluation of the pituitary gland and parasellar region is very important when a possible lesion of the gland is suspected.
Recommended imaging: dedicated pituitary magnetic resonance:
Pre- and postcontrast sagittal and coronal T1 W images, ≤ 3 mm image slice thickness with a minimal interslice gap and a small, < 23 cm, field of view. Coronal high-resolution, FSE T2 W images are important to obtain through the sella.
Optional sequence: dynamic contrast-enhanced imaging:
DCE images are acquired during the infusion of contrast at four to six locations repetitively.
Immediately after contrast administration, at 15 to 30 seconds, adenomas enhance less than the normal gland and become progressively isointense at approximately 60 seconds. On delayed imaging, 30 to 40 minutes after contrast injection, some adenomas may be hyperintense.
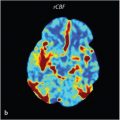
DCE-MR is a highly sensitive sequence that may detect an additional 5 to 14% of very small lesions not visualized on routine pituitary MRI. 17 , 18 , 19 , 20 , 21 As 8 to 9% of lesions are seen only on routine postcontrast imaging and not seen on the dynamic study, routine postcontrast T1 W images are still recommended in two planes following the dynamic sequence. 19
DCE is not recommended routinely due to an increased rate of false-positive results and is best reserved for cases in which the endocrinologist or neurosurgeon strongly suspect a secreting microadenoma (commonly a corticotroph), which is not apparent on the routine pituitary MRI. 13 , 22 , 23
Optional advanced MR sequences: The following techniques are described in the literature but are not currently part of most imaging centers’ dedicated pituitary protocol.
VIBE (volume interpolated breath hold): T1 W volumetric examination may yield superior resolution and improved contrast between the gland and the cavernous sinus in comparison to routine postcontrast T1 W images. It has also been reported to have greater sensitivity for detection of small adrenocorticotropic hormone (ACTH) secreting tumors. 24 , 25
VIBE with GRASP (golden angle radial sparse parallel imaging): This 3D GRE imaging technique acquires all dynamic information in a single continuous scan giving greater temporal resolution for DCE. 26
Two-plane DCE: Acquiring DCE images in two planes may provide greater confidence in diagnosing a very small microadenoma. 18
FIESTA (fast imaging employing steady-state acquisition) postcontrast for determining if pituitary mass is more likely to be soft or firm. 27
Computed tomography imaging:
Useful in patients in whom MR is contraindicated.
Superior sensitivity for calcification and therefore useful to help differentiate a craniopharyngioma from an RCC and adenoma, which rarely calcify. 28
Preferred modality for surgical navigation.
Preoperative planning sinus CT should note the following: asymmetric septation of the sphenoid sinus, attachment of septation to the optic canal, and abnormally medial position of the carotid arteries.
20.1.9 Companion Cases
Companion Case 1
Case Presentation
History
A 20-year-old man presented with a history of long-standing headaches from 6 years of age. The pituitary gland appeared prominent on routine brain MR; therefore, a dedicated pituitary MR was performed.

Imaging Analysis of Companion Case 1
Imaging Findings
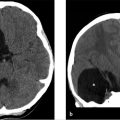
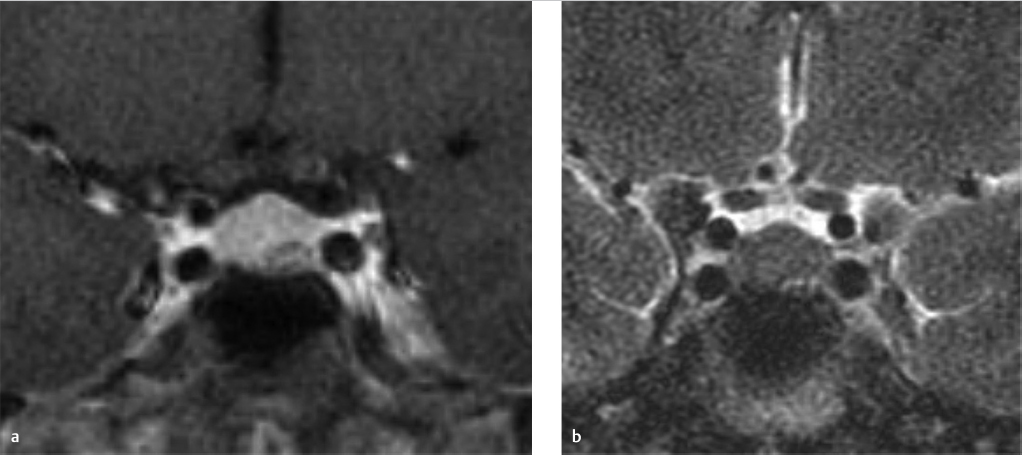
There is a kidney bean–shaped lesion in the region of the pars intermedia, which is isointense on T1 W (▶ Fig. 20.4a) and dark on T2 W (arrowhead in ▶ Fig. 20.4c) images. It appears dark on the postcontrast T1 W image (arrow in ▶ Fig. 20.4b) because it does not enhance unlike the remainder of the gland. There is no nodular enhancing component and no septation. The gland is not significantly enlarged given the size of the lesion, 3 mm × 8 mm × 6 mm. Incidental note is made of heterogeneous marrow signal in the floor of the sella, which is bright on the T1 W images (asterisk in ▶ Fig. 20.4a).

Impression
Probable Rathke′s cleft cyst and skull base hemangioma.
Differential diagnosis: See Differential Diagnosis of Intrasellar Lesions (Section 20.1.5).
Clinical Evaluation of Companion Case 1
Endocrinology Evaluation
An RCC is often an incidental finding with no additional imaging or laboratory testing needed. However, an RCC may cause pituitary hypofunction or, more rarely, coexist with a PA. 29 , 30 Therefore, testing for hypopituitarism is recommended in patients with larger cystic lesions (≥ 6 mm). 2 In this case, early morning serum cortisol, free T4, and testosterone were normal. No additional endocrine function testing is required in the absence of the development of signs or symptoms of pituitary dysfunction. As these cysts may enlarge, follow-up MRI is recommended in 1 year, with subsequently less frequent imaging in smaller lesions that are stable and more closely spaced imaging in those with larger cysts that have suprasellar extension and approach the optic chiasm. These guidelines are based on current Endocrine Society and AANS/CNS guidelines. 2 , 3 Laboratory testing and follow-up imaging guidelines vary by publishing society (see ▶ Table 20.1). The recently published American College of Radiology (ACR) guidelines do not recommend either laboratory testing for endocrine dysfunction or follow-up imaging if the lesion is a “simple cyst” that is small, without mass effect or invasion of surrounding structures. 5
Neurosurgery Evaluation
Surgical decision-making for the incidental, small RCC is similar to that of an incidental pituitary microadenoma. Observation is the treatment of choice for small, clinically asymptomatic lesions without evidence of pituitary dysfunction and in elderly patients. It is reasonable to offer surgical resection for larger RCCs in young, otherwise healthy patients. RCCs have a high rate of recurrence and aggressive surgical resection is associated with a higher rate of complication in comparison to PAs.
Imaging Pearls and Pitfalls of Rathke’s Cleft Cysts (RCC)
A thickened, enhancing cyst wall or calcification is atypical and should suggest the possibility of a craniopharyngioma. In the appropriate clinical setting, infection of an RCC has also been reported to cause increased pituitary dysfunction, a thickened, enhancing cyst wall, and restricted diffusion, all of which are not seen in an uncomplicated RCC.
Septation, fluid/fluid levels, or off-midline location suggest that the lesion is more likely a hemorrhagic PA than an RCC. 31
The differentiation between a small RCC and nonfunctioning cystic pituitary microadenoma is not always possible but is not of immediate clinical importance as neither would mandate surgical treatment.
An RCC may coexist with a PA. A lesion exerting mass effect upon a midline T1 “bright” cyst may suggest this unusual diagnosis. 7
Essential Facts about Rathke’s Cleft Cysts
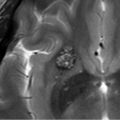
RCC is derived from embryologic remnants of Rathke’s pouch, an outpouching of the stomatodeum, in the region of the pars intermedia, which lies between the pars distalis of the adenohypophysis and the pars nervosa (see ▶ Fig. 20.5). 32
Most commonly intrasellar but may extend into the suprasellar region or, rarely, be completely suprasellar. 28
Majority have increased signal relative to cerebrospinal fluid (CSF) on both T1 W and T2 W images. 28 , 33
The presence of an intracystic nodule, high in signal on T1 W images and dark on T2 W images, has been reported in up to 77% of RCCs and is pathognomonic. 34
Homogeneous hypointense signal intensity on the T2 W images within a nonenhancing, midline pituitary cyst is highly suggestive of an RCC. 35
The “egg-in-a-cup” appearance of a well-defined oval, T1WI hyperintense mass located in a depression in the superior aspect of the pituitary gland is highly suggestive of an RCC.

Companion Case 2
Case Presentation
History
A 53-year-old woman underwent a brain MR during the evaluation of a syncopal episode. Although the normal gland was displaced to the right, there was asymmetric fullness in the left side of the gland. Therefore, a dedicated pituitary MR was performed; the images are shown in ▶ Fig. 20.6 and ▶ Fig. 20.7.


Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree