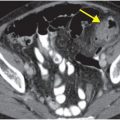
Diagnosis: Bevacizumab-associated bowel perforation
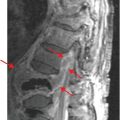
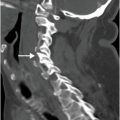
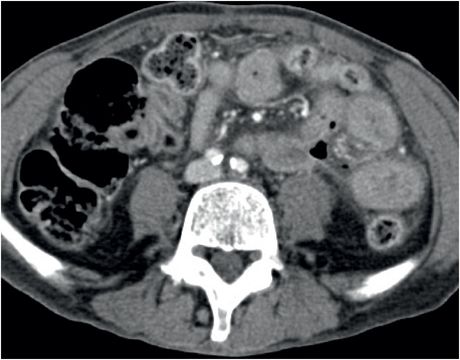
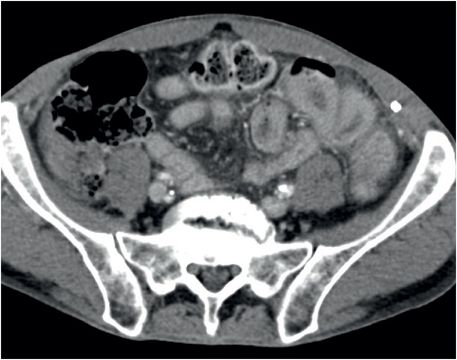
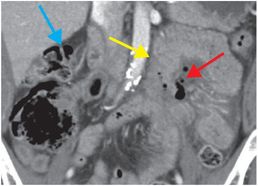
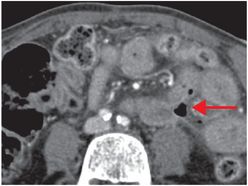
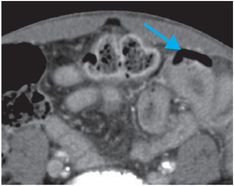
Coronal (left image) and axial (middle and right image) contrast-enhanced CT images show focal perforation at the duodenojejunal junction with diffuse wall thickening (yellow arrow) and extraluminal gas and fluid (red arrows). Scattered foci of antidependent, extraluminal gas are seen throughout the peritoneal cavity (blue arrows).
Discussion
Overview of bevacizumab
Bevacizumab (Avastin; Genentech, South San Francisco, CA) is an anti-angiogenic monoclonal antibody that inhibits the vascular endothelial growth factor (VEGF) receptor on the surface of endothelial cells.
Tumor growth and the establishment of metastases are dependent on the formation and proliferation of new blood vessels, a process that is termed angiogenesis. Angiogenesis is induced by VEGF, which promotes neovascularization and tumor growth.
By targeting the endothelial VEGF receptor, bevacizumab and other anti-VEGF agents inhibit neovascularization and abnormal blood vessel remodeling.
Bevacizumab is currently approved for use in patients with several types of advanced cancer in conjunction with standard chemotherapy, including colon cancer, breast cancer, renal cell carcinoma, non-small-cell lung cancer, and glioblastoma multiforme.
Adverse effects seen in patients on bevacizumab
VEGF is crucial to endothelial integrity and wound healing; therefore, inhibition of VEGF by bevacizumab predisposes patients to several serious and possibly fatal adverse events, including hemorrhage, neutropenia, bowel perforation, wound or anastomotic dehiscence, hypertensive crisis, and posterior reversible encephalopathy syndrome (PRES). In addition to increased risk of hemorrhage, bevacizumab also predisposes to venous and arterial thrombotic complications.
Bevacizumab-associated complications vary significantly amongst patients receiving different classes of chemotherapy, which suggests a synergistic and complex interaction between bevacizumab and specific chemotherapeutic agents.
Bevacizumab-associated bowel perforation
Bevacizumab-associated bowel perforation is a well-documented, albeit relatively uncommon, complication, occurring in approximately 0.3% of patients receiving bevacizumab, with a relative risk of 2.5 compared to similar patients not on the drug.
The etiology of bevacizumab-associated bowel perforation is not completely understood and may be secondary to treatment-induced necrosis of bowel. Other theories include damage to gastrointestinal microvasculature in association with impaired endothelial healing.
Treatment of bevacizumab-associated bowel perforation includes permanent discontinuation of bevacizumab. Depending on clinical status, either surgical intervention or conservative treatment with antibiotics and bowel rest may be performed.
Self-assessment
|
|
|
|
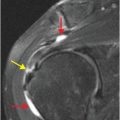
Spectrum of bevacizumab-associated complications
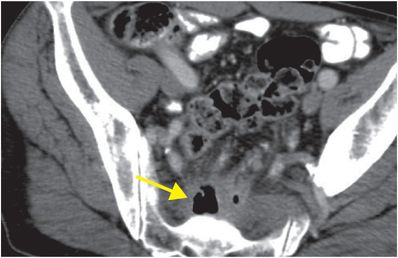
Bevacizumab-associated perforation secondary to anastomotic dehiscence
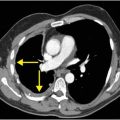
This example shows a 68-year-old male with rectal cancer, status post lower anterior resection, who presented with lower back pain. Axial (upper image) and sagittal (lower image) enhanced CT demonstrates locules of extraluminal gas in the presacral soft tissues (arrows), representing an abscess due to anastomotic dehiscence.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree