22 Incidental Glial Neoplasms
22.1 Introduction
The widespread availability of MR imaging has led to an increase in discovery of incidental gliomas. Historically, the “watch-and-wait” approach was used for incidentally detected lesions suspected to be low-grade gliomas (LGGs). It is now known that, although slow growing, these tumors often progress to high-grade malignancies. Improved microsurgical techniques for tumor resection coupled with multiple techniques for functional preservation, and a better understanding of how tumor genotype influences the natural history, have led to a paradigm shift in the management of these incidental lesions. The evidence strongly favors an early, more aggressive, function-preserving resection coupled with adjuvant chemoradiation.
In this chapter, we present the radiographic findings and management of two cases of diffuse gliomas that presented incidentally. We start with the radiographic analysis and include conventional imaging pearls that help distinguish among the different entities within the differential diagnosis. We discuss advanced brain tumor imaging techniques like diffusion tensor imaging (DTI)/tractography, perfusion and arterial spin label imaging, and MR spectroscopy, and show how they assist with preoperative diagnosis and surgical planning. Finally, we review clinical and imaging correlates of the 2016 WHO update on brain tumors, which has fundamentally altered the classification of infiltrating gliomas in adults.
22.2 Case Presentation
22.2.1 History and Physical Examination
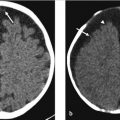
A 54-year-old man presents to the emergency room (ER) after a fall with minor superficial facial lacerations but no loss of consciousness (▶ Fig. 22.1). The ER physician found the patient was afebrile and the neurologic examination was unremarkable. A noncontrast head CT was performed.

22.2.2 Imaging Findings and Impression
There is an area of mass effect and low attenuation in the white matter of the inferior frontal gyrus (arrow in ▶ Fig. 22.1) and the anterior aspect of the insula (arrowhead in ▶ Fig. 22.1). Notably, the cortical gray matter remains normal in attenuation. This pattern of attenuation, which involves the white matter and spares the cortex, is compatible with vasogenic edema which is more suggestive of a tumor than a stroke, contusion, or encephalitis. Furthermore, the history of lack of symptoms and fever is helpful in clinically excluding an encephalitis or an acute cerebrovascular event.
22.2.3 Additional Imaging
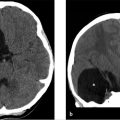
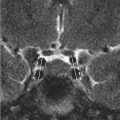
MRI of the brain with and without contrast. The fluid-attenuated inversion recovery (FLAIR) sequence (▶ Fig. 22.2a) reveals a hyperintense mass involving primarily the cortex of the pars opercularis of the frontal operculum of the inferior frontal gyrus and the anterior most aspect of the insular cortex, the short gyrus, that is fairly well defined. There is minimal mass effect upon the left basal ganglia, which otherwise appears normal. Postcontrast (▶ Fig. 22.2b), there is minimal enhancement in the region of the insula (arrow). There was no evidence of restriction on diffusion-weighted images or of susceptibility on the susceptibility-weighted image (not shown).

22.3 Clinical Evaluation
Neurosurgery was consulted while the patient was in the ER. Given the patient’s lack of fever or any other significant symptoms, contusion, an infectious process, or acute venous infarction were considered unlikely.
Additional testing needed and results: None.
22.3.1 Clinical Impression and Management
Incidentally detected mass is most likely a primary brain tumor. The patient underwent an awake craniotomy for resection with speech mapping.
Final pathology revealed anaplastic oligodendroglioma, isocitrate dehydrogenase (IDH) mutant, 1p/19q-codeleted, WHO grade III.
22.4 Differential Diagnosis
(▶ Table 22.1)
Primary brain tumor, diffuse “low-grade” glioma:
Abnormal signal and subtle mass effect involving cortical gray matter and underlying white matter with neither significant enhancement nor restriction of diffusion.
Congenital: focal cortical dysplasia (FCD):
Difficult to distinguish from LGG.
The transmantle sign, a linear area of increased signal on T2-weighted imaging (T2WI) extending from the abnormally thickening, dysplastic cortex toward the ventricle, may be present.
Congenital abnormality that presents with seizures.
No significant restriction of diffusion.
Infection: encephalitis:
Restriction of diffusion.
The most common, herpes simplex encephalitis, affects medial temporal lobes, usually bilaterally, as well as insular cortex and cingulate gyri.
Vascular: venous infarction:
Restriction of diffusion, often parasagittal high convexities.
Usually hemorrhagic and associated with venous sinus and cortical vein thrombosis.
Traumatic: contusion:
Inferior aspect of frontal lobes and anterior aspect of temporal lobes, history of trauma and hemorrhagic.
22.5 Clinical and Diagnostic Imaging Pearls and Pitfalls
Diffuse gliomas may present with seizures or other neurological abnormalities, but may also be completely asymptomatic, incidental findings as opposed to infection, contusion and venous/arterial infarction that are virtually always symptomatic with an appropriately “nonincidental” clinical history.
Diffuse, LGGs do not show significant restriction on diffusion as opposed to arterial or venous infarctions and encephalitis.
Differentiating diffuse, LGGs from FCD can be challenging as both typically present with a thickened, hyperintense cortex and varying degrees of underlying white matter signal abnormality and may also present with seizures.
The transmantle sign, inconsistently seen but highly characteristic of type IIb FCD, is a linear area of increased signal on T2WI extending from the abnormally thickening, dysplastic cortex toward the ventricle.
Magnetic resonance spectroscopy ( MRS) has been shown to be of possible value as LGGs had marked increase in Cho and a decrease in N-acetylaspartate (NAA), whereas the FCDMs (focal cortical developmental malformations) had milder Cho increase and NAA decrease.
22.6 Essential Facts about Gliomas
Gliomas are the most common type of primary adult brain tumor and have traditionally been classified and graded by histopathology according to the WHO classification system.
“LGGs” refers to WHO grade II astrocytomas and oligodendrogliomas, which are now grouped together due to similarities in their growth pattern, behavior and IDH mutation status.
The latest WHO classification of central nervous system tumors, 2016, utilizes molecular parameters in addition to histology to define tumor entities. 1 The most important of these has been identification of IDH mutations in most diffuse grade II and III gliomas and 1p19q codeletion, which is now definitional for oligodendroglial tumors.
IDH mutations occur early in pathogenesis of gliomas and are therefore often associated with TP53 and ATRX mutations in astrocytomas and 1p/19q codeletion in oligodendrogliomas. 2 , 3
22.7 Clinical Relevance of Genomic Alterations and Correlation with Conventional and Advanced Imaging Techniques
22.7.1 Isocitrate Dehydrogenase Mutations in Gliomas
Clinical relevance:
IDH mutant gliomas are more common in younger patients.
IDH mutant gliomas have a better prognosis (8.0–8.4 years median survival) compared with IDH wild type (IDHwt) (1.4–1.7 years). 4 , 5 , 6
Grade II and III IDHwt behave similarly to primary glioblastomas (GBMs)—they have a very poor prognosis. All IDHwt tumors should be evaluated for GBM features. 1
Aggressive surgical resection beyond enhancing margins benefits IDH mutant tumors. 7
Conventional imaging:
IDH-mutated LGGs are found to have a significant preferential localization in the frontal lobe and in the limbic structures. 8
IDH-mutated grade II and III astrocytomas are more likely to be confined to a single lobe as compared to IDHwt tumors, which are more likely multifocal.
IDH-mutated gliomas often have a well-defined boundary on MRI, they may have a larger nonenhancing component than IDHwt tumors, and are more likely to have tumor-related cystic components.
Advanced imaging:
Diffusion: A lower apparent diffusion coefficient (ADC) reflects greater restriction of water molecule movement within higher grade tumors due to their increased cellularity.
Lower ADC values are found in IDHwt GBMs and grade III gliomas. 9 , 10 , 11 , 12 , 13 Low ADC values, independent of tumor grade, correlate with poor survival in grade III and IV gliomas. 14
Minimum ADC (ADCmin) and rADC (relative ADC) are significantly higher in grade II and III astrocytomas with the IDH mutation. 15
Diffusion restriction has been found to be an independent predictor of IDH mutation status in grade II gliomas and has independent prognostic value. ADCmin, threshold < 0.9 × 10 to 3 mm2/s, had 91% sensitivity and 76% specificity for IDH wt and when combined with IDH status was better in predicting progression-free and overall survival better than mutation status alone. 16
MRS:
MRS has been used to directly detect 2-HG (2-hydroxyglutarate), an oncometabolite produced in tumors with an IDH mutation; currently, however, it cannot replace pathological markers for determining the IDH mutation status. 17
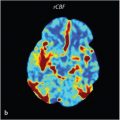
Perfusion imaging provides a measure of tumor neovascularity that has been used to index malignant potential.
However, the data are conflicting—some studies show no definite distinction between IDHwt and IDH-mutated tumors using relative cerebral blood volume (rCBV) mean or maximum rCBV, 16 while others show a lower rCBV max in mutant tumors. 10 , 15
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree