Benign Neoplasms |
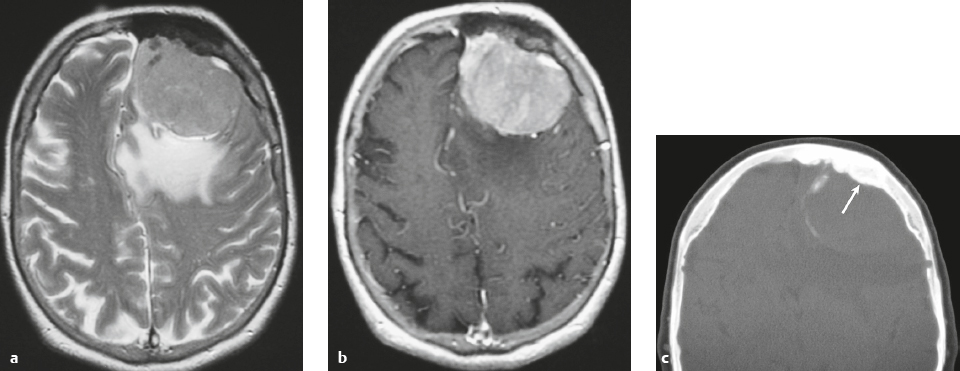
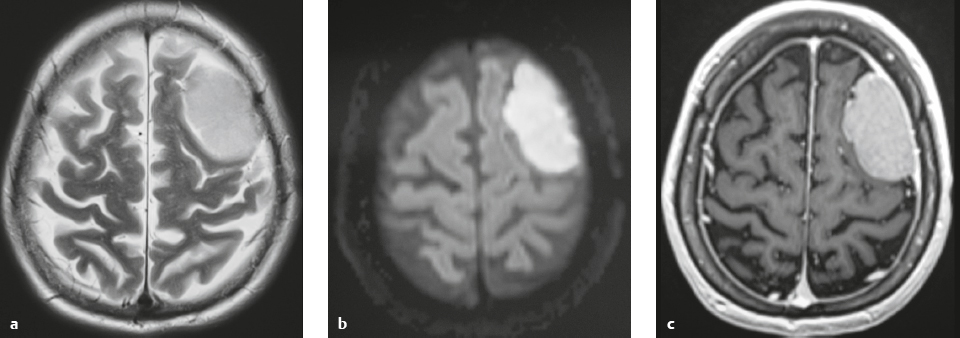
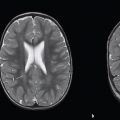
Meningioma ( Fig. 3.1, Fig. 3.2, and Fig. 3.3 ) |
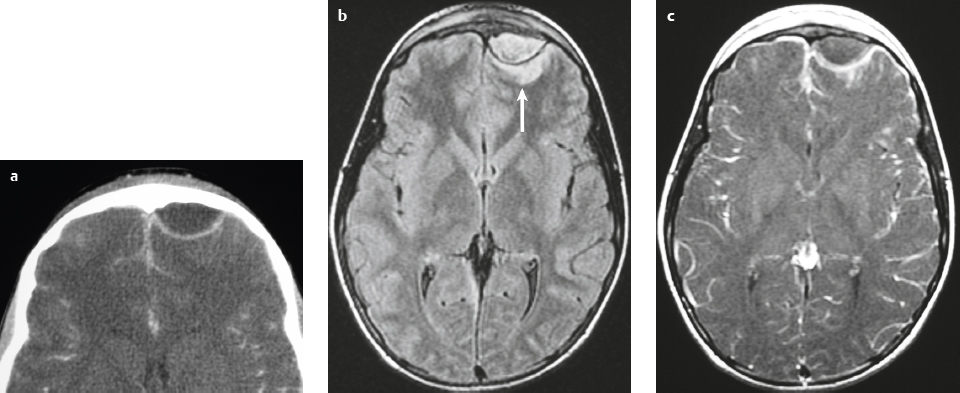
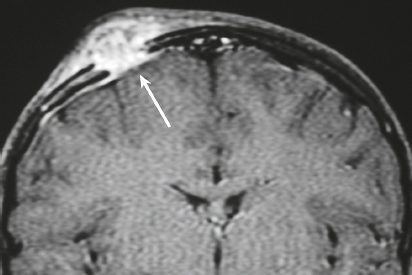
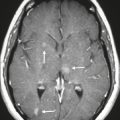
Well-circumscribed extra-axial dura-based lesions. Locations: supra- > infratentorial, parasagittal > convexity > sphenoid ridge > parasellar > posterior fossa > optic nerve sheath > intraventricular.
MRI: Dura-based tumors with intermediate signal on T1-weighted imaging, intermediate to slightly high signal on T2-weighted imaging, usually with prominent gadolinium contrast enhancement, often with a dural tail, ± calcifications. Intratumoral hemorrhage and cystic or necrotic foci can occur in 15%. Can result in compression of adjacent brain parenchyma, encasement of arteries, and compression of dural venous sinuses. Rarely invasive/malignant.
Diffusion-weighted imaging/diffusion tensor imaging: ADC values vary among the different subtypes of meningioma. Some tumors can show restricted diffusion, although these findings can be seen with both benign and atypical tumors.
Magnetic resonance spectroscopy can show elevated alanine (1.5 ppm), lactate, choline, and glutamine/glutamate levels, and reduced N- acetylaspartate (NAA).
CT: Tumors have intermediate attenuation with or without calcifications, with or without hyperostosis, and usually show prominent contrast enhancement. |
Meningiomas are the most common extra-axial tumors, accounting for up to 26% of primary intracranial tumors. Annual incidence is 6 per 100,000, typically in adults (> 40 years old) and in women > men. Composed of neoplastic meningothelial (arachnoid or arachnoid cap) cells. Meningiomas are usually solitary and sporadic, but can also occur as multiple lesions in patients with neurofibromatosis type 2. Eighty percent of meningiomas are benign (WHO grade I), although 15% have atypical features (WHO grade II) and ~ 5% have anaplastic histologic features (WHO grade III). Can occur secondary to radiation treatment, with latencies ranging from 19 to 35 years.
Classified into different subtypes, such as meningothelial, fibrous (fibroblastic), transitional (mixed), psammomatous, angiomatous, atypical, and anaplastic. The most common intracranial types of meningioma are meningothelial, fibrous, and transitional types. Usually show immunoreactivity to epithelial membrane antigen (EMA) and vimentin. Secretory meningiomas are typically immunoreactive to CEA. Associated cytogenetic findings of deletion of chromosome 22. Mutations in the NF2 tumor suppressor gene on chromosome 22 have been found in 60% of sporadic meningiomas. |
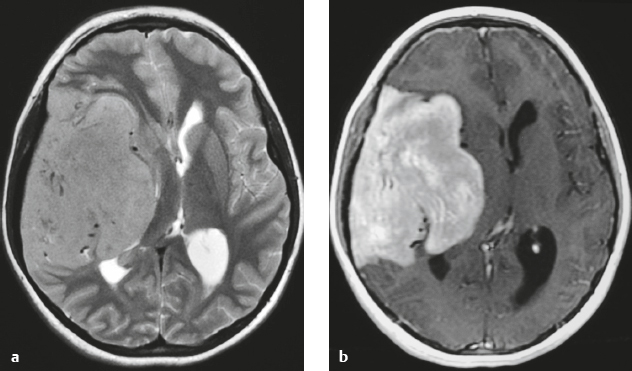
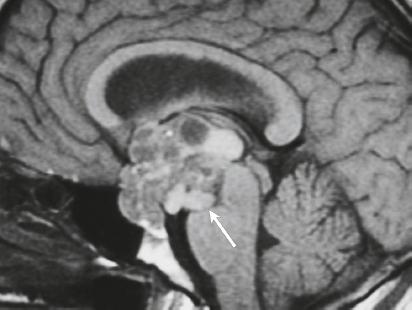
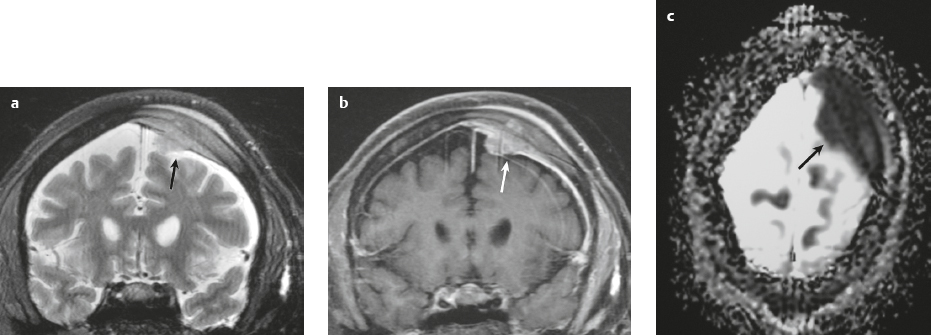
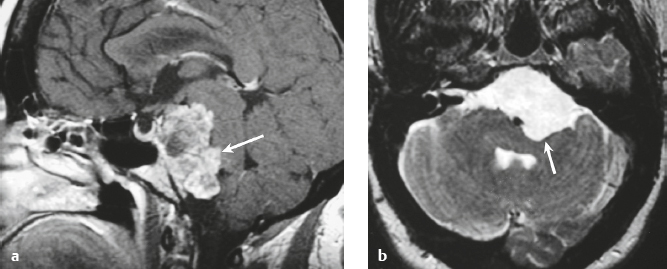
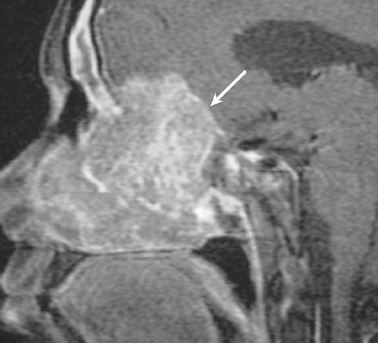
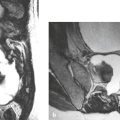
Hemangiopericytoma ( Fig. 3.4 ) |
MRI: Solitary dura-based tumors ranging from 2 to 7 cm in diameter that have low-intermediate signal on T1-weighted imaging, intermediate to slightly high signal on T2-weighted imaging, and usually prominent gadolinium contrast enhancement, often with a dural tail, ± calcifications. Intratumoral hemorrhage and cystic or necrotic foci can occur in 30%.
Magnetic resonance spectroscopy: May show elevated choline peak. Relative ratios of myo-inositol, glucose, and glutathione with respect to glutamate are higher in hemangiopericytomas than in meningiomas.
CT: Tumors have intermediate attenuation with or without calcifications and usually show prominent contrast enhancement. |
Rare (WHO grade II) neoplasms that account for 0.4% of primary intracranial tumors and are 50 times less frequent than meningiomas. Tumors are composed of closely packed cells with scant cytoplasm and round, ovoid, or elongated nuclei with moderately dense chromatin. Numerous slitlike vascular channels are seen in these tumors that are lined by flattened endothelial cells, ± zones of necrosis. Immunoreactive to vimentin (85%) and factor XIIIa (80–100%), and variably to Leu-7. and CD34. Associated with abnormalities involving chromosome 12. Typically occur in young adults (mean age = 43 years) and in males > females. Sometimes referred to as angioblastic meningioma or meningeal hemangiopericytoma, these tumors arise from vascular cells—pericytes. Recur and metastasize more frequently than meningiomas. |
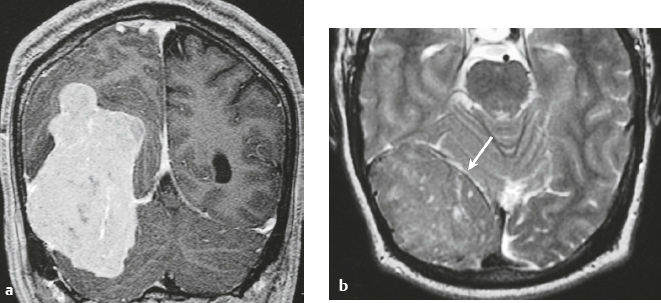
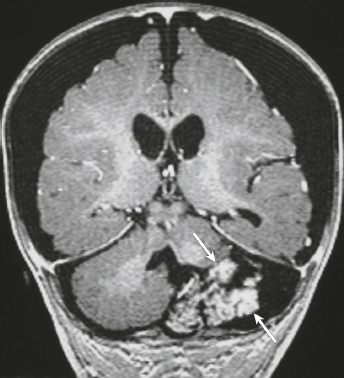
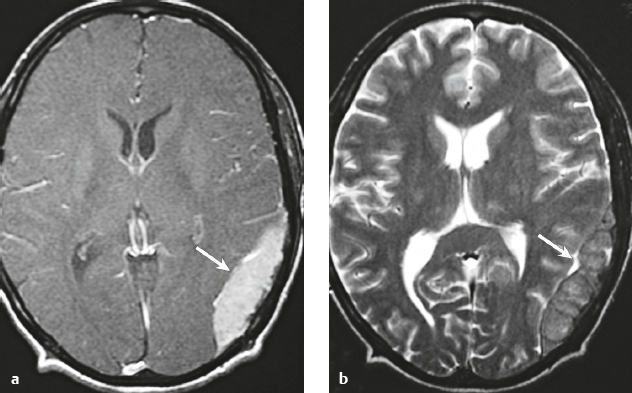
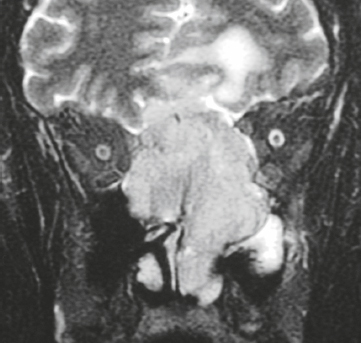
Meningioangiomatosis ( Fig. 3.5 ) |
MRI: Lesions usually involve the superficial brain tissue and leptomeninges and have low-intermediate signal on T1-weighted imaging, high signal on T2-weighted imaging, and gyriform high signal on FLAIR, ±low signal from calcifications. Typically show gadolinium contrast enhancement.
Magnetic resonance spectroscopy: Choline peaks can be elevated.
CT: Nodular lesions with low-intermediate attenuation involving the superficial brain, ±calcifications, ± perilesional edema, and usually show contrast enhancement. |
Rare benign hamartomatous lesions involving the leptomeninges and adjacent cerebral cortex. Lesions contain numerous thickened blood vessels surrounded by sheaths of well-differentiated meningothelial cells and concentric layers of collagen bundles, ± psammoma bodies, and typically have a low MIB-1 proliferative rate (< 1%). Mean age =18 years. Most common locations are temporal and frontal lobes. Lesions are solitary in sporadic cases and multiple in patients with neurofibromatosis type 2. |
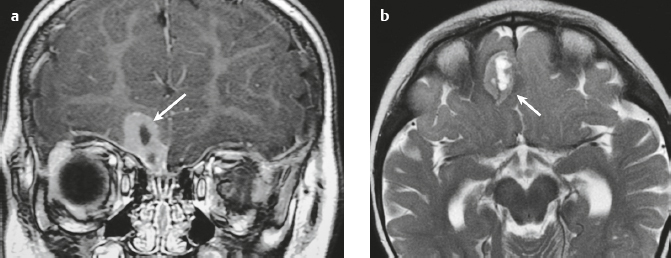
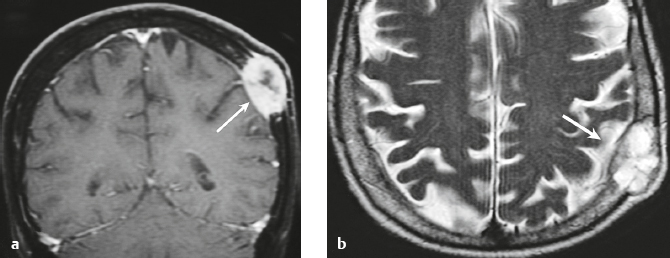
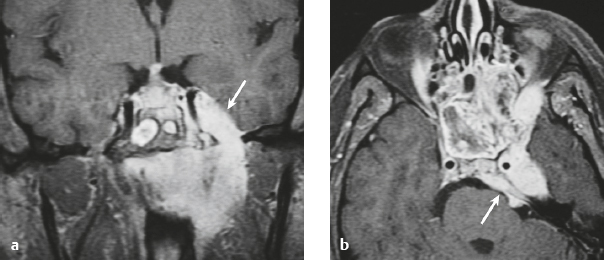
Schwannoma ( Fig. 3.6 ) |
MRI: Circumscribed or lobulated lesions with low-intermediate signal on T1-weighted imaging and high signal on T2-weighted imaging and fat-suppressed T2-weighted imaging. Usually show prominent gadolinium contrast enhancement. High signal on T2-weighted imaging and gadolinium contrast enhancement can be heterogeneous in large lesions due to cystic degeneration, fibrosis, and/or hemorrhage. Schwannomas involving the temporal bone include those from CN V (trigeminal nerve cistern/Meckel′s cave), CN VI (Dorello canal), CN VII and CN VIII (IAC and CPA cistern), CN IX, CN X, and CN XI (jugular foramen). CT: Circumscribed or lobulated lesions, intermediate attenuation, + contrast enhancement. Large lesions can have cystic degeneration and/or hemorrhage. |
Schwannomas are benign encapsulated tumors that contain differentiated neoplastic Schwann cells. Most commonly occur as solitary sporadic lesions. Multiple schwannomas are often associated with neurofibromatosis type 2 (NF2), which is an autosomal dominant disease involving a gene at chromosome 22q12. In addition to schwannomas, patients with NF2 can also have multiple meningiomas and ependymomas. Schwannomas represent 8% of primary intracranial tumors and 29% or primary spinal tumors.
The incidence of NF2 is 1/37,000 to 1/50,000 newborns. Age at presentation is 22 to 72 years (mean age = 46 years). Peak incidence is in the fourth to sixth decades. In NF2, many patients present in the third decade with bilateral vestibular schwannomas. |
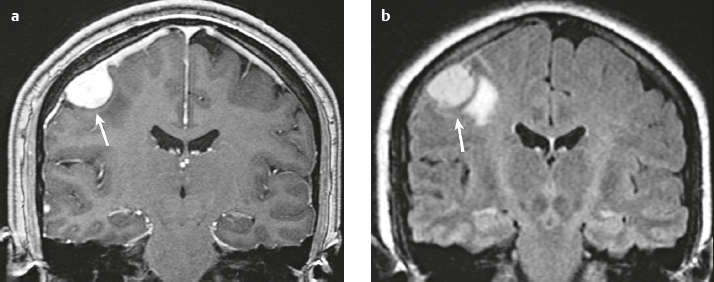
Neurofibroma |
MRI: Solitary neurofibromas appear as circumscribed or lobulated extra-axial lesions with low-intermediate signal on T1-weighted imaging and intermediate-high signal on T2-weighted imaging, + prominent gadolinium contrast enhancement. High signal on T2-weighted imaging and gadolinium contrast enhancement can be heterogeneous in large lesions. Plexiform neurofibromas appear as curvilinear and multinodular lesions involving multiple nerve branches and have low to intermediate signal on T1-weighted imaging and intermediate, slightly high to high signal on T2-weighted imaging and fat-suppressed T2-weighted imaging, with or without bands or strands of low signal. Lesions usually show gadolinium contrast enhancement.
CT: Ovoid or fusiform lesions with low-intermediate attenuation. Lesions can show contrast enhancement. Often erode adjacent bone. |
Benign nerve sheath tumors that contain mixtures of Schwann cells, perineural-like cells, and interlacing fascicles of fibroblasts associated with abundant collagen. Unlike schwannomas, neurofibromas lack Antoni A and B regions and cannot be separated pathologically from the underlying nerve. Most frequently occur as sporadic, localized, solitary lesions, less frequently as diffuse or plexiform lesions. Multiple neurofibromas are typically seen in neurofibromatosis type 1, which is an autosomal dominant disorder (1/2,500 births) from mutations in the neurofibromin gene on chromosome 17q11.2. Represents the most common type of neurocutaneous syndrome and is associated with neoplasms of the central and peripheral nervous systems (optic gliomas, astrocytomas, plexiform and solitary neurofibromas) and skin (café-au-lait spots, axillary and inguinal freckling). Also associated with meningeal and skull dysplasias, as well as hamartomas of the iris (Lisch nodules). |
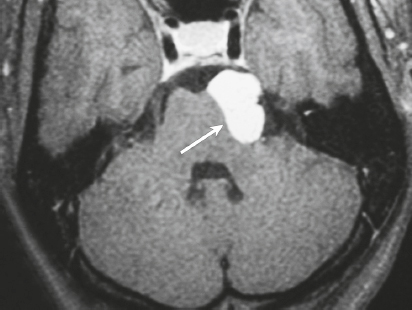
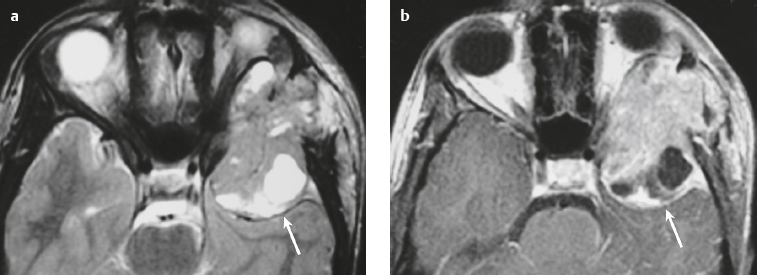
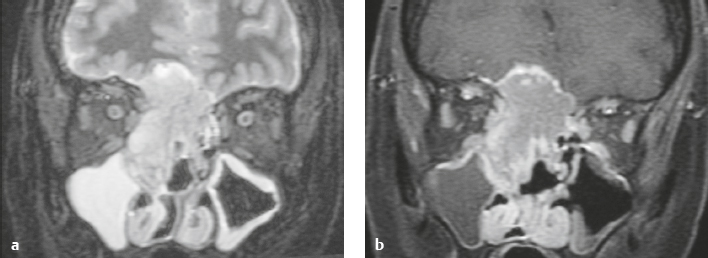
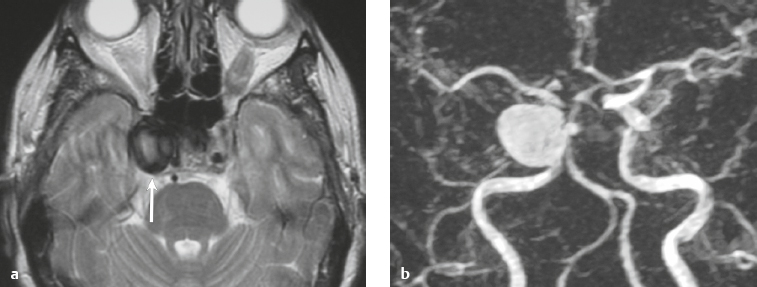
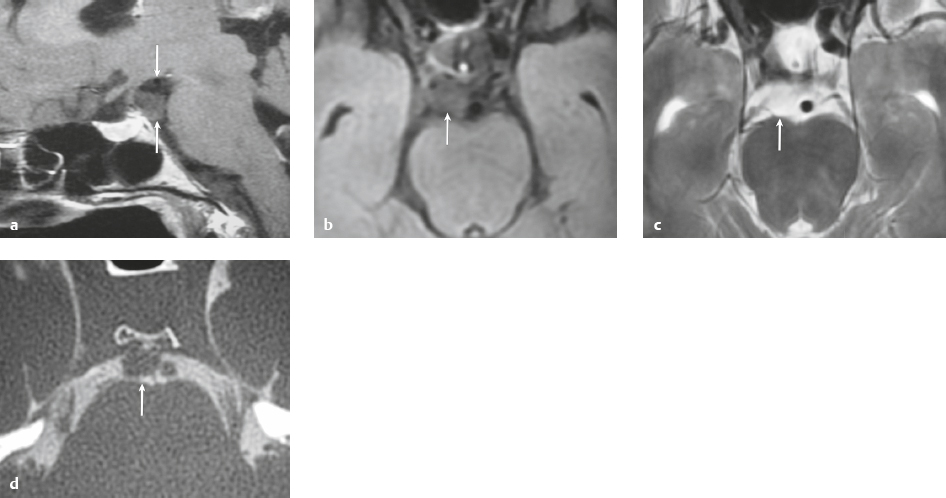
Paraganglioma ( Fig. 3.7 ) |
Ovoid or fusiform lesions with low-intermediate attenuation.
MRI: Spheroid or lobulated lesion with intermediate signal on T1-weighted imaging and intermediate-high signal on T2-weighted imaging and fat-suppressed T2-weighted imaging, ± tubular zones of flow voids. Usually show prominent gadolinium contrast enhancement, ± foci of high signal on T1-weighted images from mucin or hemorrhage, ± peripheral rim of low signal (hemosiderin) on T2-weighted imaging. CT: Lesions can show contrast enhancement. Often erode adjacent bone. |
Benign encapsulated neuroendocrine tumors that arise from neural crest cells associated with autonomic ganglia (paraganglia) throughout the body. These lesions are also referred to as chemodectomas and are named according to location (glomus jugulare, tympanicum, vagale). Paragangliomas represent 0.6% of head and neck tumors and 0.03% of all neoplasms. |
Benign mesenchymal non-meningothelial tumors |
MRI and CT findings of these lesions are dependent on their histologic features. |
Benign mesenchymal tumors (WHO grade I) can rarely occur as solitary lesions involving the meninges and skull. Lesions include lipoma, angiolipoma, hibernoma, solitary fibrous tumor, leiomyoma, rhabdomyoma, chondroma, and osteoma. |
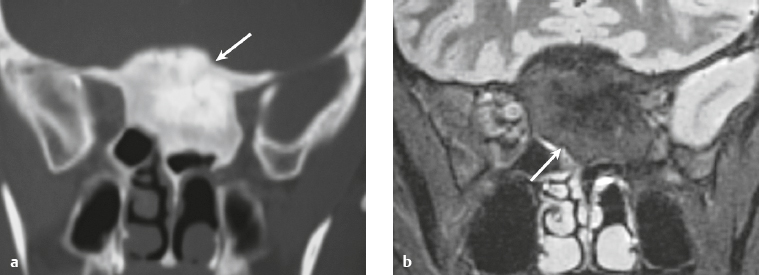
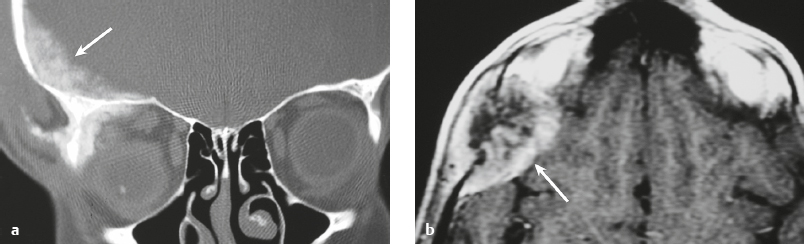
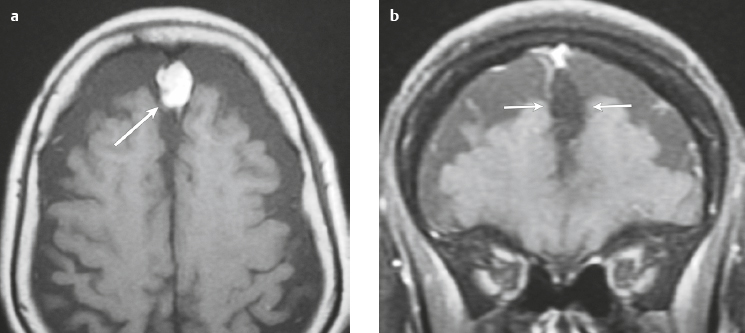
Osteoma ( Fig. 3.8 ) |
MRI: Osteomas typically appear as well-circumscribed zones of dense bone with low signal on T1-weighted imaging, FLAIR imaging, T2-weighted imaging, and fat-suppressed T2-weighted imaging.
CT: Dense round or ovoid lesions with well-circumscribed margins that are contiguous with cortical bone, and inner or outer table of the skull is usually smooth or lobulated. |
Primary osseous tumors composed of dense lamellar, woven, and/or compact cortical bone and usually located at the surface of bones. Usually occur in Gardner′s syndrome, which is an autosomal dominant disorder that is associated with intestinal polyposis, fibromas, and desmoid tumors. |
Pituitary adenoma ( Fig. 3.9 ) |
Microadenomas (< 10 mm): Commonly have intermediate signal on T1- and T2-weighted imaging occasionally have high signal on T2-weighted imaging, ± cyst, ± hemorrhage, ± necrosis. Typically enhance less than normal pituitary tissue—often best seen with dynamic early phase imaging.
Macroadenomas (> 10 mm): Commonly have intermediate signal on T1- and T2-weighted imaging similar to gray matter, ± necrosis, ± cyst, ± hemorrhage. Usually have prominent gadolinium contrast enhancement, extension into suprasellar cistern with waist at diaphragma sella, ± extension into cavernous sinus, occasionally invading skull base. |
Common, benign, slow-growing tumors representing ~ 50% of sellar/parasellar neoplasms in adults. Can be associated with endocrine abnormalities related to oversecretion of hormones (prolactin > nonsecretory type > growth hormone > ACTH > others). Prolactinomas occur in females > males. Growth hormone tumors occur in males > females. |
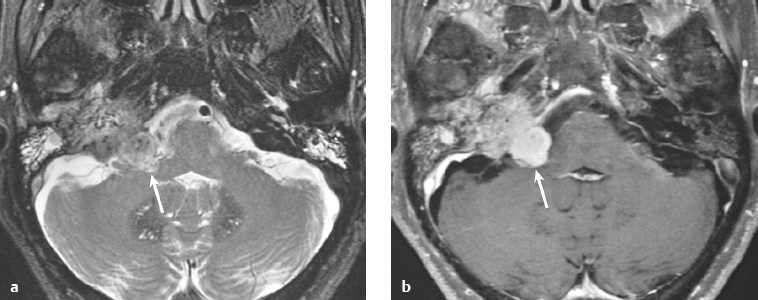
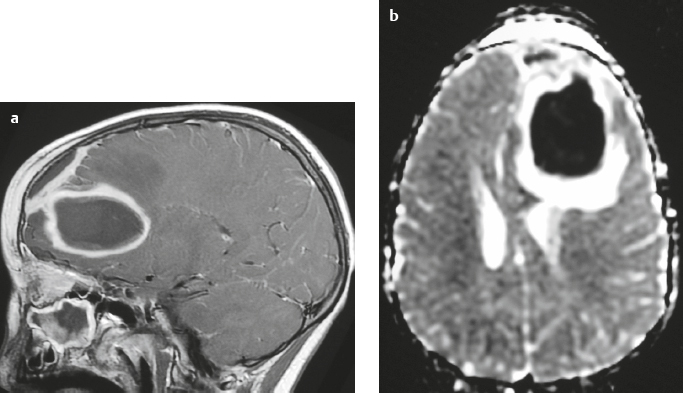
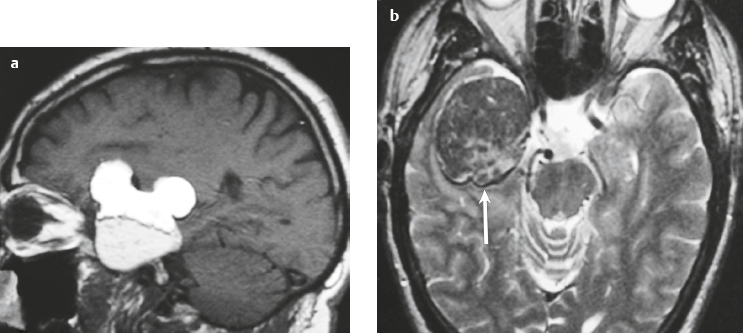
Craniopharyngioma ( Fig. 3.10 ) |
Circumscribed, lobulated lesions; both suprasellar and intrasellar location > suprasellar > intrasellar locations. Lesions can contain cysts, lipid components, and calcifications.
MRI: Lesions can have variable low, intermediate, and/or high signal on T1- and T2-weighted imaging, ± nodular or rim patterns of gadolinium contrast enhancement.
CT: Circumscribed, lobulated lesions; variable low, intermediate, and/or high attenuation; ± nodular or rim patterns of contrast enhancement ± calcifications. |
Usually histologically benign but locally aggressive lesions arising from squamous epithelial rests along Rathke′s cleft. Lesions contain cords and and lobules of squamous epithelium, pallisaded columnar epithelium, cystic cavities containing squamous debris and/or cholesterol, and piloid gliosis with Rosenthal fibers. Mutations of the β-catenin gene in 70%. Accounts for 1.2 to 4.6% of all intracranial tumors, with an incidence of 2 cases per million per year. Occurs in children (10 years old) and adults (> 40 years old) and in males = females. |
Choroid plexus papilloma ( Fig. 3.11 ) |
MRI: Circumscribed and/or lobulated lesions with papillary projections, intermediate signal on T1-weighted imaging and mixed intermediate-high signal on T2-weighted imaging, usually with prominent gadolinium contrast enhancement, ±calcifications. Locations: atrium of lateral ventricle (children) > fourth ventricle (adults), rarely other locations. such as third ventricle. Associated with hydrocephalus from CSF overproduction or mechanical obstruction.
CT: Circumscribed and/or lobulated lesions with papillary projections, intermediate attenuation, and usually prominent contrast enhancement, ± calcifications. |
Rare benign (WHO grade I) intraventricular neoplasms derived from choroid plexus epithelium in which a single layer of cuboidal or columnar epithelial cells with round/oval nuclei overlies fibrovascular connective tissue in frondlike patterns. Occur in lateral ventricle (50%), fourth ventricle/CP angle (40%), third ventricle (5%), and multiple ventricles (5%). Occur in children and adults. Tumors in the lateral ventricles account for up to 80% of cases in patients less than 20 years old. Lesions in the fourth ventricle are most common in adults. Tumors have very low mitotic activity. Immunoreactive to cytokeratins, vimentin, podoplanin, S-100, and transthyretin. Can be cured by surgical resection. |
Malignant Neoplasms |
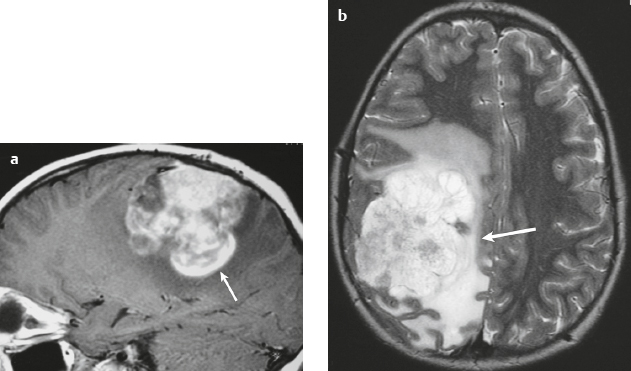
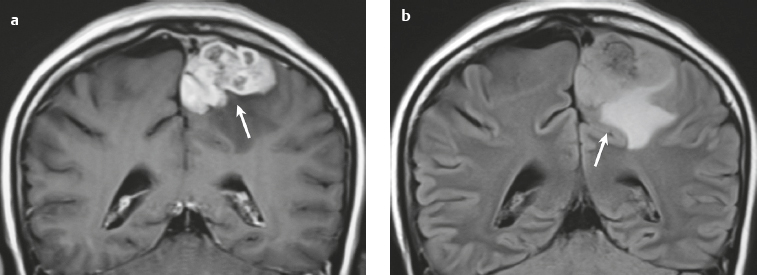
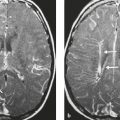
Malignant meningioma ( Fig. 3.12 and Fig. 3.13 ) |
MRI: Dura-based tumors with intermediate signal on T1-weighted imaging and intermediate to slightly high signal on T2-weighted imaging, usually with prominent gadolinium contrast enhancement, ± calcifications. Malignant meningiomas are often large and may have irregular margins, with brain invasion and peritumoral edema. Difusion-weighted imaging/diffusion tensor imaging: ADC values vary among the different subtypes of meningioma. Some tumors can show restricted diffusion, although these findings can be seen with both benign and malignant tumors.
Magnetic resonance spectroscopy: Can show elevated alanine (1.5 ppm), lactate, choline, and glutamine/glutamate peaks, and reduced N-acetylaspartate (NAA). MRS cannot reliably differentiate benign from malignant meningiomas.
CT: Tumors have intermediate attenuation with or without calcifications, with or without hyperostosis, and usually show prominent contrast enhancement. |
Eighty percent of meningiomas are benign (WHO grade I), although 15% have atypical features (WHO grade II) and ~ 5% have anaplastic/malignant histologic features (WHO grade III). Atypical and anaplastic/malignant meningiomas are associated with recurrence rates at 5 years of 40% and 50–80%, respectively. |
Anaplastic hemangiopericytoma |
MRI: Solitary, lobulated, dura-based tumors that have low-intermediate signal on T1-weighted imaging, mixed intermediate, slightly high, and high signal on T2-weighted imaging, and usually prominent heterogeneous gadolinium contrast enhancement. Intratumoral hemorrhage and cystic or necrotic foci are often present, ± dural tail, ± calcifications, ± bone destruction, ± peritumoral edema.
CT: Tumors have intermediate attenuation with or without calcifications, and usually show prominent contrast enhancement. |
Anaplastic hemangiopericytomas (WHO grade III) have high degrees of nuclear atypia with mitotic activities of greater than 5 mitoses per 10 HPF. Ki-67 activity is > 15%. Recur and metastasize more frequently than WHO grade II hemangiopericytomas. |
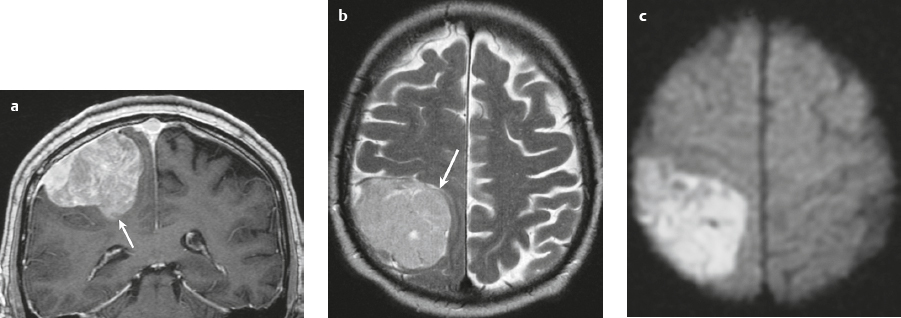
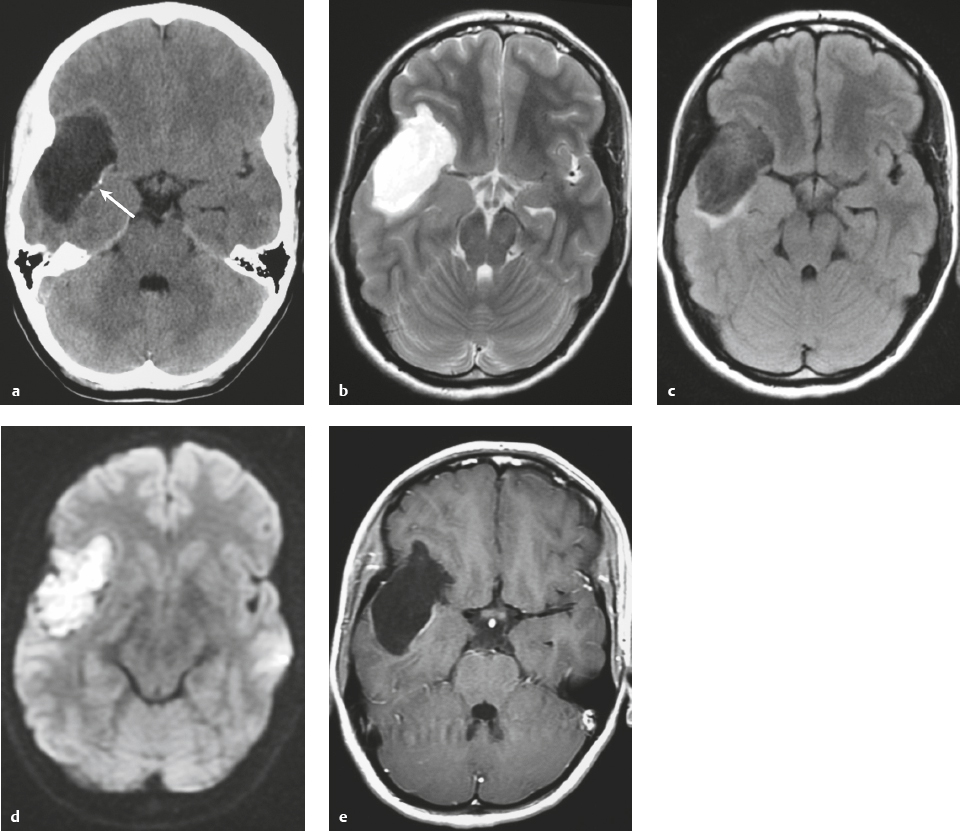
Metastatic tumor ( Fig. 3.14 ) |
MRI: Circumscribed spheroid lesion in bone, dura, leptomeninges, and/or choroid plexus. Often has low-intermediate signal on T1-weighted imaging and intermediate-high signal on T2-weighted imaging, ± hemorrhage, calcifications, and cysts. Variable gadolinium contrast enhancement.
CT: Lesions usually have low-intermediate attenuation, ± hemorrhage, calcifications, and cysts. Variable contrast enhancement, ± bone destruction, ± compression of neural tissue or vessels. Leptomeningeal tumor often best seen on postcontrast images. |
Represent ~ 33% of intracranial tumors, usually from extracranial primary neoplasm in adults > 40 years old. Primary tumor source: lung > breast > GI > GU > melanoma. Can occur as single or multiple well-circumscribed or poorly defined lesions involving the skull, dura, leptomeninges, and/or choroid plexus. Metastatic tumor may cause variable destructive or infiltrative changes in single or multiple sites. |
Lymphoma ( Fig. 3.15 ) |
In immunocompetent patients, primary CNS lymphoma (PCNSL) occurs as a solitary focal or infiltrating lesion in 65% of cases.
MRI: Single or multiple well-circumscribed or poorly defined lesions involving the skull, dura, and/or leptomeninges; low-intermediate signal on T1-weighted imaging, intermediate-high signal on T2-weighted imaging, usually + gadolinium contrast enhancement, ± bone destruction. Leptomeningeal tumor often best seen on postcontrast images.
CT: CNS lymphoma can have intermediate attenuation or can be hyperdense related to a high nuclear/cytoplasm ratio. FDG PET/CT can show elevated uptake in PCNSL and in immunocompromised patients can be used to distinguish lymphoma from toxoplasmosis brain lesions, which have decreased FDG uptake. |
Primary CNS lymphoma is more common than secondary, usually in adults > 40 years old. Accounts for 5% of primary brain tumors. Incidence currently ranges from 0.8 to 1.5% of primary intracranial tumors. Prior elevated incidence of 6% in patients with AIDS has been reduced with effective antiviral therapy. B-cell lymphoma is more common than T-cell lymphoma. MRI features of primary and secondary lymphoma of brain overlap. Intracranial lymphoma can involve the leptomeninges in secondary lymphoma > primary lymphoma. |
Plasmacytoma/myeloma ( Fig. 3.16 ) |
Multiple (myeloma) or single (plasmacytoma) well-circumscribed or poorly defined lesions involving the skull and dura.
MRI: Well-circumscribed or poorly defined lesions involving the skull and dura with low-intermediate signal on T1-weighted imaging and intermediate-high signal on T2-weighted imaging. Usually show gadolinium contrast enhancement, + bone destruction.
CT: Lesions have low-intermediate attenuation, usually + contrast enhancement, + bone destruction. |
In multiple myeloma, the malignancy is comprised of proliferating antibody-secreting plasma cells derived from single clones. Multiple myelomas are primarily located in bone marrow. A solitary myeloma or plasmacytoma is an infrequent variant in which a neoplastic mass of plasma cells occurs at a single site of bone or soft tissue. In the United States, 14,600 new cases occur each year. Multiple myeloma is the most common primary neoplasm of bone in adults. Median age at presentation = 60 years. Most patients are older than 40 years.
Tumors occur in the vertebrae > ribs > femur > iliac bone > humerus > craniofacial bones > sacrum > clavicle > sternum > pubic bone > tibia. |
Malignant mesenchymal non-meningothelial tumors ( Figs. 3.17 ) |
MRI and CT findings of these lesions are dependent on their histologic features. Malignant tumors may be associated with invasion of adjacent brain, bone, and/or leptomeninges. |
Malignant mesenchymal tumors (WHO grades III and IV) can rarely occur as solitary lesions involving the meninges and skull. Lesions include: malignant fibrous histiocytoma, fibrosarcoma, rhabdomyosarcoma, leiomyosarcoma, liposarcoma, chondrosarcoma, osteosarcoma, Ewing′s sarcoma, and angiosarcoma. |
Chordoma ( Fig. 3.18 ) |
Well-circumscribed lobulated lesions along dorsal surface of clivus, vertebral bodies, or sacrum, + localized bone destruction.
MRI: Lesions have low-intermediate signal on T1-weighted images and high signal on T2-weighted images, + gadolinium contrast enhancement (usually heterogeneous). Locally invasive, associated with bone erosion/destruction, encasement of vessels (usually without luminal narrowing), and nerves. Commonly located in skull base-clivus, usually in the midline.
CT: Lesions have low-intermediate attenuation, ± calcifications from destroyed bone carried away by tumor, + contrast enhancement. |
Chordomas are rare, locally aggressive, slow-growing, low to intermediate grade malignant tumors derived from ectopic notochordal remnants along the axial skeleton. Chondroid chondromas (5 to 15% of all chordomas) have both chordomatous and chondromatous differentiation. Chordomas that contain sarcomatous components are referred to as dedifferentiated chordoma or sarcomatoid chordoma (5% of all chordomas). Chordomas account for 2–4% of primary malignant bone tumors, 1–3% of all primary bone tumors, and less than 1% of intracranial tumors. The annual incidence has been reported to be 0.18 to 0.3 per million. Dedifferentiated chordomas or sarcomatoid chordomas account for less than 5% of all chordomas. For cranial chordomas, patients’ mean age = 37 to 40 years. |
Chondrosarcoma ( Fig. 3.19 ) |
Lobulated lesions with bone destruction at synchondroses.
MRI: Lesions have low-intermediate signal on T1-weighted imaging, high signal on T2-weighted imaging, ± matrix mineralization with low signal on T2-weighted images, + gadolinium contrast enhancement (usually heterogeneous). Locally invasive, associated with bone erosion/destruction, encasement of vessels and nerves. Commonly located in skull base petro-occipital synchondrosis, usually off midline.
CT: Lesions have low-intermediate attenuation associated with localized bone destruction, ± chondroid matrix calcifications, + contrast enhancement. |
Chondrosarcomas are malignant tumors containing cartilage formed within sarcomatous stroma. Chondrosarcomas can contain areas of calcification/mineralization, myxoid material, and/or ossification. Chondrosarcomas rarely arise within synovium. Chondrosarcomas represent from 12 to 21% of malignant bone lesions, 21 to 26% of primary sarcomas of bone, 9 to14% of all bone tumors, 6% of skull base tumors, and 0.15% of all intracranial tumors. MRI is important for planning surgical approaches. |
Osteogenic sarcoma ( Fig. 3.20 ) |
Destructive lesions involving the skull base or calvarium.
MRI: Tumors often have poorly defined margins and commonly extend from the marrow through destroyed bony cortex into adjacent soft tissues. Tumors usually have low-intermediate signal on T1-weighted imaging. Zones of low signal often correspond to areas of tumor calcification/mineralization and/or necrosis. On T2-weighted imaging, zones of necrosis typically have high signal, whereas mineralized zones usually have low signal. Tumors can have variable MRI signal on T2-weighted imaging and fat-suppressed T2-weighted imaging depending upon the relative proportions, distributions, and locations of calcified/mineralized osteoid, chondroid, fibroid, and hemorrhagic and necrotic components. Tumors may have low, low-intermediate, and intermediate to high signal on T2-weighted imaging and fat-suppressed T2-weighted imaging. After gadolinium contrast administration, osteosarcomas typically show prominent enhancement in nonmineralized/noncalcified portions.
CT: Tumors have low-intermediate attenuation, usually + matrix mineralization/ossification, and often show contrast enhancement (usually heterogeneous). |
Osteosarcomas are rare malignant tumors comprised of proliferating neoplastic spindle cells that produce osteoid and/or immature tumoral bone. Occur in children as primary tumors, and in adults they are associated with Paget′s disease, irradiated bone, chronic osteomyelitis, osteoblastoma, giant cell tumor, fibrous dysplasia. Locally invasive, with high metastatic potential. |
Ewing′s sarcoma ( Fig. 3.21 ) |
MRI: Destructive lesion involving the skull base, with low-intermediate signal on T1-weighted imaging, mixed low, intermediate, and high signal on T2-weighted imaging, ± malignant periosteal reaction with low signal on T2-weighted imaging, + gadolinium contrast enhancement (usually heterogeneous).
CT: Destructive lesion involving the skull base, with low-intermediate attenuation, and can show contrast enhancement (usually heterogeneous). |
Malignant primitive tumor of bone comprised of undifferentiated small cells with round nuclei. Accounts for 6 to 11% of primary malignant bone tumors, 5 to 7% of primary bone tumors. Usually occurs between the ages of 5 and 30 years, in males > in females. Ewing′s sarcomas commonly have translocations involving chromosomes 11 and 22: t (11;22) (q24:q12) which results in fusion of the FL1-1 gene at 11q24 to the EWS gene at 22q12. Locally invasive, with high metastatic potential. Rare lesions involve the skull base. |
Sinonasal squamous cell carcinoma ( Fig. 3.22 ) |
Destructive lesions arising in the nasal cavity and paranasal sinuses, ±intracranial extension via bone destruction or perineural spread.
MRI: Destructive lesions in the nasal cavity, paranasal sinuses, and nasopharynx, ±intracranial extension via bone destruction or perineural spread. Intermediate signal on T1-weighted imaging and intermediate-slightly high signal on T2-weighted imaging, often with gadolinium contrast enhancement. Large lesions (± necrosis and/or hemorrhage).
CT: Tumors have intermediate attenuation and mild contrast enhancement and are large (±necrosis and/or hemorrhage). |
Malignant epithelial tumors originating from the mucosal epithelium of the paranasal sinuses (maxillary, 60%; ethmoid, 14%; sphenoid and frontal sinuses, 1%) and nasal cavity (25%). Include both keratinizing and nonkeratinizing types. Account for 3% of malignant tumors of the head and neck. Occur in adults usually > 55 years old, and in males > females. Associated with occupational or other exposure to tobacco smoke, nickel, chlorophenols, chromium, mustard gas, radium, and material in the manufacture of wood products. |
Nasopharyngeal carcinoma ( Fig. 3.23 ) |
MRI: Invasive lesions in the nasopharynx (lateral wall/fossa of Rosenmüller and posterior upper wall) ± intracranial extension via bone destruction or perineural spread. Intermediate signal on T1-weighted imaging and intermediate-slightly high signal on T2-weighted imaging, often with gadolinium contrast enhancement. Large lesions (± necrosis and/or hemorrhage).
CT: Tumors have intermediate attenuation and mild contrast enhancement and are large (± necrosis and/or hemorrhage). |
Carcinomas arising from the nasopharyngeal mucosa with varying degrees of squamous differentiation. Subtypes include squamous cell carcinoma, nonkeratinizing carcinoma (differentiated and undifferentiated), and basaloid squamous cell carcinoma. Occurs at higher frequency in Southern Asia and Africa than in Europe and the Americas. Peak ages are 40–60 years. Occurs two to three times more frequently in men than in women. Associated with Epstein-Barr virus, diets containing nitrosamines, and chronic exposure to tobacco smoke, formaldehyde, chemical fumes, and dust. |
Adenoid cystic carcinoma ( Fig. 3.24 ) |
MRI: Destructive lesions in the paranasal sinuses, nasal cavity, and nasopharynx that can have intracranial extension via bone destruction or perineural spread. Intermediate signal on T1-weighted imaging and intermediate-high signal on T2-weighted imaging, with variable mild, moderate, or prominent gadolinium contrast enhancement.
CT: Tumors have intermediate attenuation and variable mild, moderate, or prominent contrast enhancement. |
Basaloid tumor comprised of neoplastic epithelial and myoepithelial cells. Morphologic tumor patterns include tubular, cribiform, and solid. Accounts for 10% of epithelial salivary neoplasms. Most commonly involves the parotid, submandibular, and minor salivary glands (palate, tongue, buccal mucosa and floor of the mouth, other locations). Perineural tumor spread common, ± facial nerve paralysis. Usually occurs in adults > 30 years old. Solid type has the worst prognosis. Up to 90% die within 10–15 years after diagnosis. |
Esthesioneuroblastoma ( Fig. 3.25 ) |
MRI: Locally destructive lesions with low-intermediate signal on T1-weighted images and intermediate-high signal on T2-weighted images, + prominent gadolinium enhancement. Locations: superior nasal cavity, ethmoid air cells with occasional extension into the other paranasal sinuses, orbits, anterior cranial fossa, and cavernous sinuses.
CT: Locally destructive lesions with low-intermediate attenuation, usually showing contrast enhancement. Locations: superior nasal cavity, ethmoid air cells with occasional extension into the other paranasal sinuses, orbits, anterior cranial fossa, and cavernous sinuses. |
These tumors, also referred to as olfactory neuroblastoma, arise from olfactory epithelium in the superior nasal cavity. Tumors consist of immature neuroblasts with variable nuclear pleomorphism, mitoses, and necrosis. Tumor cells occur in a neurofibrillary intercellular matrix. Bimodal occurrence in adolescents (11–20 years old) and adults (50–60 years old), and occur in males more than in females. |
Hemorrhage |
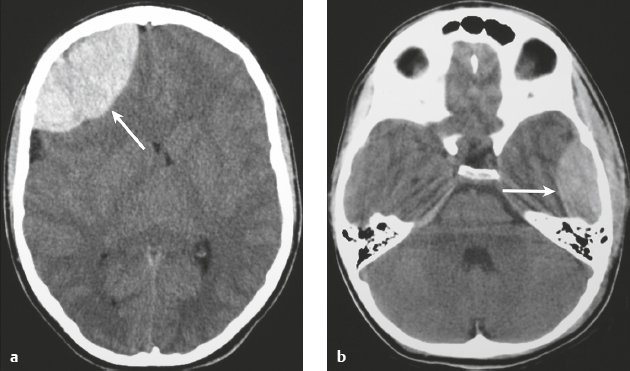
Epidural hematoma ( Fig. 3.26 and Fig. 3.27 ) |
MRI: Biconvex extra-axial hematoma located between the skull and dura (displaced dura has low signal on T2-weighted imaging), ± edema (high signal on T2-weighted imaging involving the displaced brain parenchyma), ± subfalcine or uncal herniation. The signal of the hematoma itself depends on its age, size, hematocrit, and oxygen tension. Hyperacute: Intermediate signal on T1-weighted imaging and intermediate-high signal on T2-weighted imaging. Acute: Low-intermediate signal on T1-weighted imaging and high signal on T2-weighted imaging. Early Subacute: High signal on T1-weighted imaging and low signal on T2-weighted imaging. Subacute: High signal on T1- and T2-weighted imaging.
CT: Biconvex extra-axial hematoma located between the skull and dura (displaced dura has high attenuation), ± low-attenuation edema involving the displaced brain parenchyma, ± subfalcine or uncal herniation. The CT attenuation of the hematoma depends on its age, size, hematocrit, and oxygen tension. |
Epidural hematomas usually result from trauma/tearing of an epidural artery (often the middle meningeal artery) or dural venous sinus, ± skull fracture. Epidural hematomas do not cross cranial sutures. |
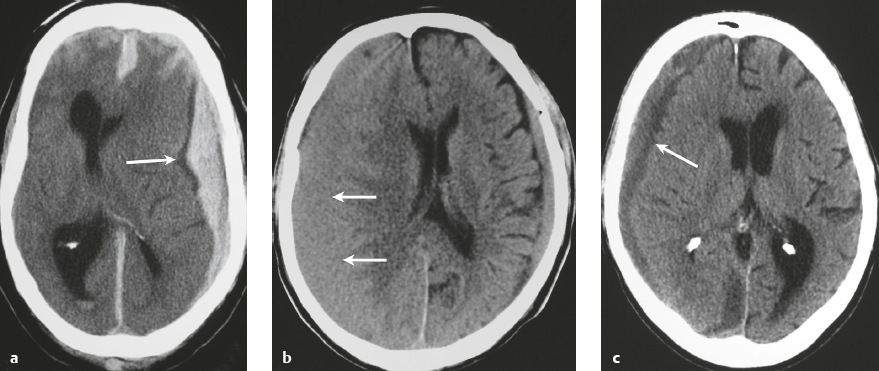
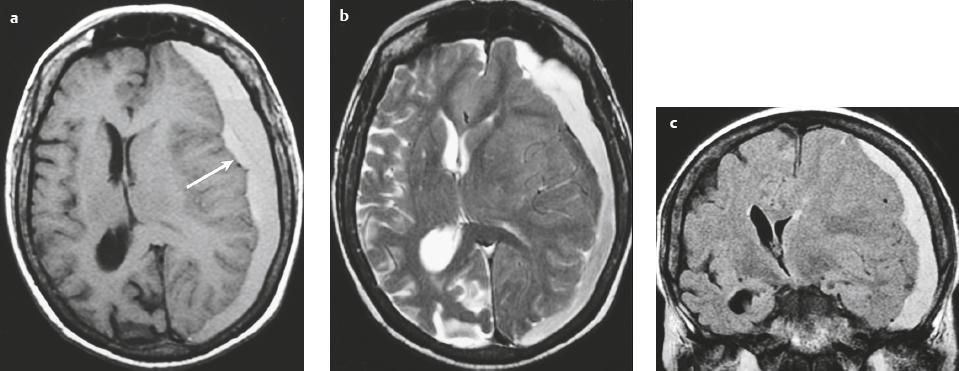
Subdural hematoma ( Fig. 3.28 and Fig. 3.29 ) Hyperacute hematoma Acute hematoma Subacute hematoma Chronic hematoma |
Crescentic extra-axial hematoma located in the potential space between the inner margin of the dura and outer margin of the arachnoid membrane, ± edema (low attenuation on CT and high signal on T2-weighted imaging) involving the displaced brain parenchyma, ± subfalcine and uncal herniation. The CT attenuation and MRI signal of the hematoma depend on its age, size, hematocrit, and oxygen tension.
Hyperacute hematoma
CT: Can have high or mixed high, intermediate, and/or low attenuation.
MRI: Intermediate signal on T1-weighted imaging and intermediate-high signal on T2-weighted imaging.
Acute hematoma CT: Can have high or mixed high, intermediate, and/or low attenuation.
MRI: Low-intermediate signal on T1-weighted imaging, low signal on T2-weighted imaging.
Subacute hematoma
CT: Can have intermediate attenuation (isodense to brain) and/or low-intermediate attenuation.
MRI: High signal on T1- and T2-weighted imaging.
Chronic hematoma
CT: Usually have low attenuation (hypodense to brain).
MRI: Variable, often low-intermediate signal on T1-weighted imaging, high signal on T2-weighted imaging, ± gadolinium contrast enhancement of collection and organizing neomembrane. Mixed MRI signal can result if rebleeding occurs into chronic collection. |
Subdural hematomas usually result from trauma/stretching/tearing of cortical veins where they enter the subdural space to drain into dural venous sinuses, ± skull fracture. Subdural hematomas do cross sites of cranial sutures. |
Ossified hematoma |
MRI: Thin or irregular peripheral zones with low signal from calcifications; peripheral and/or central ossification surrounding collections with variable low, intermediate, and/or high signal on T1- and T2-weighted imaging, ± gadolinium contrast enhancement of collection and organizing neomembrane. Mixed MRI signal can result if rebleeding occurs into chronic collection.
CT: Usually has dense thin or irregular peripheral calcifications, and/or peripheral and central ossification surrounding collections with variable low, intermediate, and/or high attenuation. |
Chronic subdural or epidural hematomas can rarely become calcified or ossified, and they are referred to as armored brain. Surgical removal of symptomatic lesions can be difficult because of dense adhesion to brain surface and/or dura. |
Infection/Inflammation |
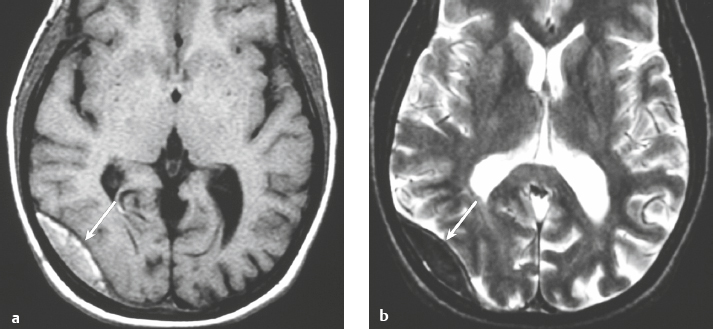
Epidural abscess or subdural empyema ( Fig. 3.30 and Fig. 3.31 ) |
MRI: Epidural or subdural collections with low signal on T1-weighted imaging, high signal on T2-weighted imaging, and thin linear peripheral zones of gadolinium contrast enhancement.
CT: Epidural or subdural collections with low attenuation and linear peripheral zones of contrast enhancement. |
Often results as a complication of sinusitis (usually frontal), meningitis, otitis media, ventricular shunts, or surgery. Can be associated with venous sinus thrombosis and venous cerebral or cerebellar infarctions, cerebritis, or brain abscess. Mortality is 30%. |
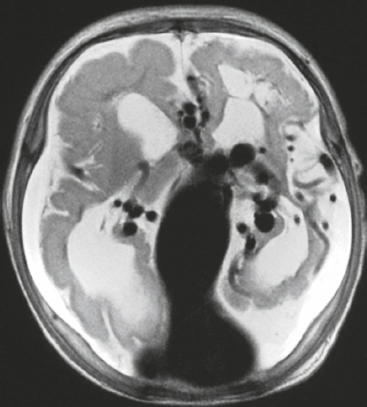
Langerhans’ cell histiocytosis/eosinophilic granuloma ( Fig. 3.32 ) |
MRI: Single or multiple circumscribed soft tissue lesions in the marrow of the skull associated with focal bony destruction/erosion with extension extracranially, intracranially, or both. Lesions usually have low-intermediate signal on T1-weighted imaging, mixed intermediate-slightly high signal on T2-weighted imaging, + gadolinium contrast enhancement, ± enhancement of the adjacent dura.
CT: Single or multiple circumscribed soft tissue lesions in the marrow of the skull associated with focal bony destruction/erosion with extension extracranially, intracranially, or both. Lesions usually have low-intermediate attenuation, + contrast enhancement, ± enhancement of the adjacent dura. |
Single lesion: Commonly seen in males > females, < 20 years old, with proliferation of histiocytes in medullary cavity and localized destruction of bone with extension in adjacent soft tissues.
Multiple lesions: Associated with Letterer-Siwe disease (lymphadenopathy and hepatosplenomegaly) in children < 2 years old and Hand-Schüller-Christian disease (lymphadenopathy, exophthalmos, and diabetes insipidus) in children 5–10 years old. |
Vascular Lesions |
Arterial aneurysm ( Fig. 3.33 and Fig. 3.34 ) Saccular aneurysm Giant aneurysm Fusiform aneurysm |
Saccular aneurysm
MRI: Focal well-circumscribed zone of signal void on T1- and T2-weighted imaging, with variable mixed signal if thrombosed.
CT: Focal well-circumscribed zone of intermediate-high attenuation, + contrast enhancement in nonthrombosed portion of aneurysm, easily visualized on CTA.
Giant aneurysm
MRI: Focal well-circumscribed structure with layers of low, intermediate, and high signal on T2-weighted imaging secondary to layers of thrombus of different ages, as well as a zone of signal void representing a patent lumen if present. On T1-weighted imaging, layers of intermediate and high signal can be seen as well as a zone of signal void.
CT: Focal well-circumscribed structure with layers of low, intermediate, and/or high attenuation secondary to layers of thrombus of different ages, as well as a zone of contrast enhancement representing a patent lumen if present, ± wall calcifications present.
Fusiform aneurysm
MRI: Elongated and ectatic arteries, variable intraluminal MR signal related to turbulent or slowed blood flow or partial/complete thrombosis.
CT: Elongated and ectatic arteries with intermediate attenuation, ± calcifications, variable contrast enhancement related to turbulent or slowed blood flow or partial/complete thrombosis. |
Abnormal fusiform or focal dilatation of artery secondary to acquired/degenerative etiology, polycystic disease, connective tissue disease, atherosclerosis, trauma, infection (mycotic), oncotic involvement, arteriovenous malformation, vasculitis, and drugs. Focal aneurysms are also referred to as saccular aneurysms, which typically occur at arterial bifurcations and are multiple in 20% of cases. Saccular aneurysms > 2.5 cm in diameter are referred to as giant aneurysms. Fusiform aneurysms are often related to atherosclerosis or collagen vascular disease (Marfan syndrome, Ehlers-Danlos syndrome, etc.). Dissecting aneurysms form when hemorrhage occurs in the arterial wall from incidental or significant trauma. |
Dural arteriovenous malformations (AVMs) |
MRI: Dural AVMs contain multiple, tortuous, tubular flow voids on T1- and T2-weighted imaging. The venous portions often show gadolinium enhancement. Gradient echo MR images and MRA using time-of-flight or phase-contrast techniques show flow signal in patent portions of the vascular malformation and areas of venous sinus occlusion or recanalization. Usually not associated with mass effect unless there is recent hemorrhage or venous occlusion.
CT: Dural AVMs contain multiple, tortuous, contrast-enhancing vessels on CTA at the site of a recanalizing thrombosed dural venous sinus. Usually not associated with mass effect. |
Dural AVMs are usually acquired lesions resulting from thrombosis or occlusion of an intracranial venous sinus with subsequent recanalization resulting in direct arterial to venous sinus communications. Occur in transverse and sigmoid venous sinuses > cavernous sinus > straight and superior sagittal sinuses. |
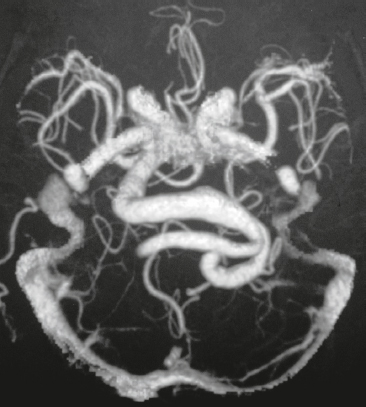
Vein of Galen aneurysm ( Fig. 3.35 ) |
MRI: Multiple, tortuous, tubular flow voids on T1- and T2-weighted imaging involving choroidal and thalamoperforate arteries, internal cerebral veins, vein of Galen (aneurysmal formation), straight and transverse venous sinuses, and other adjacent veins and arteries. The venous portions often show gadolinium enhancement. Gradient echo MR images and MRA using time-of-flight or phase-contrast techniques show flow signal in patent portions of the vascular malformation.
CT: Multiple, tortuous, contrast-enhancing vessels involving choroidal and thalamoperforate arteries, internal cerebral veins, vein of Galen (aneurysmal formation), straight and transverse venous sinuses, and other adjacent veins and arteries. The venous portions often show contrast enhancement. CTA shows contrast enhancement in patent portions of the vascular malformation. |
Heterogeneous group of vascular malformations with arteriovenous shunts and dilated deep venous structures draining into and from an enlarged vein of Galen, ± hydrocephalus, ± hemorrhage, ± macrocephaly, ± parenchymal vascular malformation components, ± seizures. High-output congestive heart failure in neonates. |
Tumorlike Lesions |
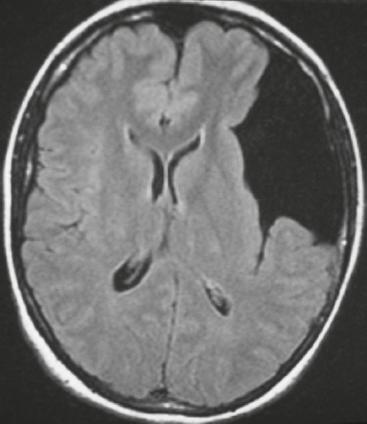
Arachnoid cyst ( Fig. 3.36 ) |
MRI: Well-circumscribed extra-axial lesions with low signal on T1-weighted imaging, FLAIR, and diffusion-weighted imaging and high signal on T2-weighted imaging similar to CSF, as well as no gadolinium contrast enhancement. Common locations: anterior middle cranial fossa > suprasellar/quadrigeminal cisterns > frontal convexities > posterior cranial fossa.
CT: Well-circumscribed extra-axial lesions with low attenuation and no contrast enhancement. |
Nonneoplastic congenital, developmental, or acquired extra-axial lesions filled with CSF, usually with mild mass effect on adjacent brain, in supratentorial > infratentorial locations, and in males > females, ± related clinical symptoms. |
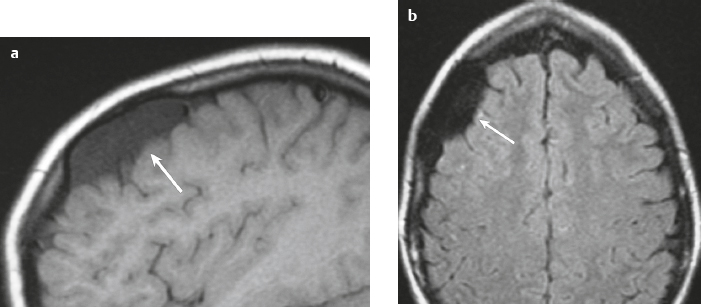
Leptomeningeal cyst ( Fig. 3.37 ) |
MRI: Well-circumscribed extra-axial lesions with low signal on T1-weighted imaging and high signal on T2-weighted imaging similar to CSF and no gadolinium contrast enhancement. Associated with erosion of the adjacent skull.
CT: Well-circumscribed extra-axial lesions with low attenuation similar to CSF and no contrast enhancement. |
Nonneoplastic extra-axial lesions filled with CSF thought to be secondary to trauma with dural tear/skull fracture, usually with mild mass effect on adjacent brain and progressive erosion of adjacent skull. Occasionally presents as a scalp lesion, and occurs in children > adults. |
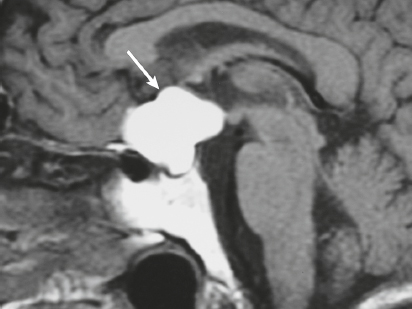
Rathke′s cleft cyst ( Fig. 3.38 ) |
MRI: Well-circumscribed lesions with variable low, intermediate, or high signal on T1- and T2-weighted imaging. On T1-weighted imaging, two-thirds have high signal and one-third have low signal; on T2-weighted imaging, one-half have high signal, one-fourth have low signal, and one-fourth have intermediate signal, – gadolinium centrally, ± thin peripheral enhancement. Lesion locations: intrasellar, 50%; suprasellar, 25%; intra- and suprasellar, 25%.
CT: Well-circumscribed lesion with variable low, intermediate, or high attenuation and no contrast enhancement. |
Uncommon sellar/juxtasellar benign cystic lesions containing fluid with variable amounts of protein, mucopolysaccharide, and/or cholesterol that arise from epithelial rests of the craniopharyngeal duct. |
Epidermoid ( Fig. 3.39 ) |
MRI: Well-circumscribed spheroid or multilobulated extra-axial ectodermal-inclusion cystic lesions with low-intermediate signal on T1-weighted imaging, high signal on T2-weighted imaging and diffusion weighted imaging (restricted diffusion), mixed low, intermediate, or high signal on FLAIR images, and no gadolinium contrast enhancement. Often insinuate along CSF pathways, causing chronic deformation of adjacent neural tissue (brainstem, brain parenchyma).
CT: Well-circumscribed spheroid or multilobulated extra-axial ectodermal-inclusion cystic lesions with low-intermediate attenuation. |
Nonneoplastic congenital or acquired extra-axial off-midline lesions filled with desquamated cells and keratinaceous debris, usually with mild mass effect on adjacent brain, and in infratentorial > supratentorial locations. Occur in adults, in males = females, ± related clinical symptoms. Commonly located in posterior cranial fossa (cerebellopontine angle cistern) > parasellar/middle cranial fossa. |
Dermoid ( Fig. 3.40 ) |
MRI: Well-circumscribed spheroid or multilobulated extra-axial lesions, usually with high signal on T1-weighted images, variable low, intermediate, and/or high signal on T2-weighted imaging, and no gadolinium contrast enhancement, ± fluid/fluid or fluid/debris levels.
CT: Well-circumscribed spheroid or multilobulated extra-axial lesions, usually with low attenuation, ± fat/fluid or fluid/debris levels. |
Nonneoplastic congenital or acquired ectodermal-inclusion cystic lesions filled with lipid material, cholesterol, desquamated cells, and keratinaceous debris, usually with mild mass effect on adjacent brain. Occur in adults, in males slightly > females, ± related clinical symptoms. Can cause chemical meningitis if dermoid cyst ruptures into the subarachnoid space. Commonly located at or near midline, supra- > infratentorial. |
Neurenteric cyst ( Fig. 3.41 ) |
MRI: Well-circumscribed, spheroid, intradural extra-axial lesions, with low, intermediate, or high signal on T1- and T2-weighted imaging, and usually no gadolinium contrast enhancement.
CT: Circumscribed intradural extra-axial structures with low-intermediate attenuation. Usually no contrast enhancement, |
Neurenteric cysts are malformations in which there is a persistent communication between the ventrally located endoderm and the dorsally located ectoderm secondary to developmental failure of separation of the notochord and foregut. Obliteration of portions of a dorsal enteric sinus can result in cysts lined by endothelium, fibrous cords, or sinuses. Observed in patients < 40 years old. Locations: thoracic > cervical > posterior cranial fossa > craniovertebral junction > lumbar; usually midline in position and often ventral to the spinal cord or brainstem. Associated with anomalies of the adjacent vertebrae and clivus. |
Lipoma ( Fig. 3.42 ) |
MRI: Lipomas have signal isointense to subcutaneous fat on T1-weighted images (high signal); and on T2-weighted images. Signal suppression occurs with frequency-selective fat saturation techniques or with a short time to inversion recovery (STIR) method. Typically there is no gadolinium enhancement or peripheral edema.
CT: Lipomas have CT attenuation similar to subcutaneous fat, typically with no contrast enhancement or peripheral edema. |
Benign fatty lesions resulting from congenital malformation, often located in or near the midline, and may contain calcifications and/or traversing blood vessels. |
Dural calcification and ossification ( Fig. 3.43 ) |
MRI: Zones of calcification usually have low signal on T1-weighted imaging, gradient recalled echo (GRE), and T2-weighted imaging. Zones of ossification can have a low-signal border on T1- and T2-weighted imaging and a central zone of fat marrow signal.
CT: Calcification as well as zones of ossification can be seen in one or more sites of the intracranial dura. |
Calcification and ossification can occur at single or multiple sites in the intracranial dura from metaplasia. Typically they are incidental findings. |
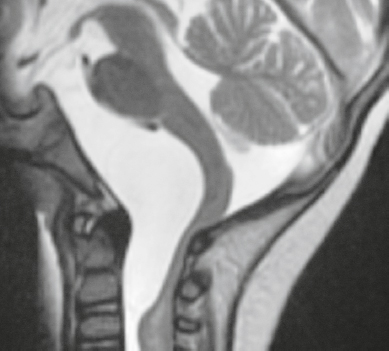
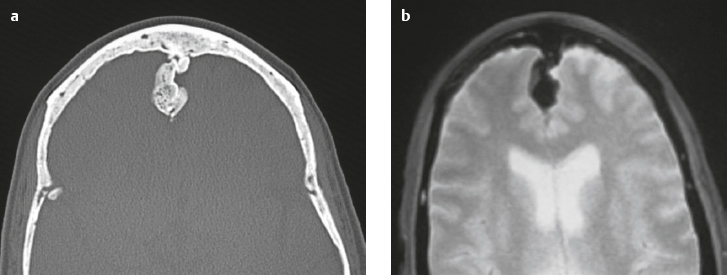
Ecchordosis physaliphora ( Fig. 3.44 ) |
MRI: Circumscribed lesion ranging in size from 1 to 3 cm, with low signal on T1-weighted imaging, low to intermediate signal on FLAIR, and high signal on T2-weighted imaging. Typically shows no gadolinium contrast enhancement.
CT: Lesions typically have low attenuation, ± remodeling/erosion of adjacent bone, ± small calcified bone stalk. |
Congenital benign hamartoma composed of gelatinous tissue with physaliphorous cell nests derived from ectopic vestigial notochord. Incidence at autopsy ranges from 0.5 to 5%. Usually located intradurally dorsal to the clivus and dorsum sella within the prepontine cistern, and rarely dorsal to the upper cervical spine or sacrum. Rarely occurs as an extradural lesion. Results from an ectopic notochordal remnant or from extension of extradural notochord at the dorsal wall of the clivus through the adjacent dura into the subarachnoid space. Typically are asymptomatic and observed as an incidental finding in patients between 20 and 60 years old. |