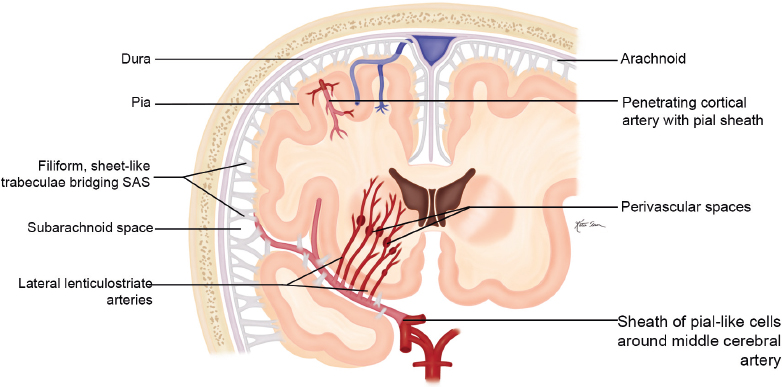
Congenital/Developmental |
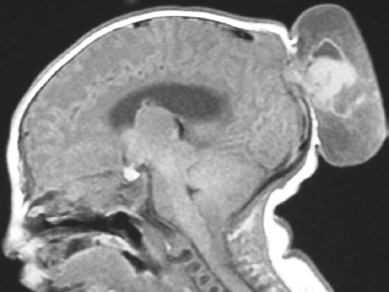
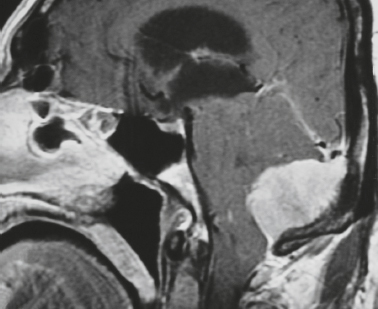
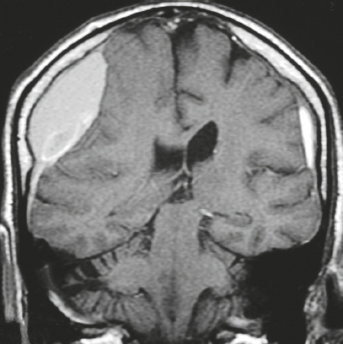
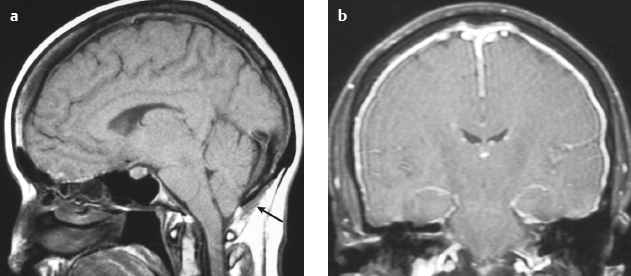
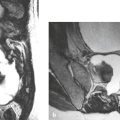
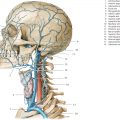
Cephaloceles (meningoceles or meningoencephaloceles) ( Fig. 4.2 ) |
Defect in skull through which there is either herniation of meninges and CSF (meningocele) or of meninges, CSF/ventricles, and brain tissue (meningoencephalocele). |
Congenital malformation involving lack of separation of neuroectoderm from surface ectoderm, with resultant localized failure of bone formation. Occipital location most common in patients in Western hemisphere, frontoethmoidal location most common site in Southeast Asians. Other sites include parietal and sphenoid bones. Cephaloceles can also result from trauma or surgery. |
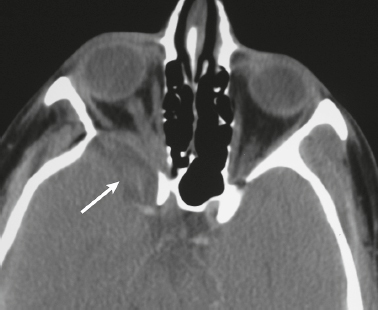
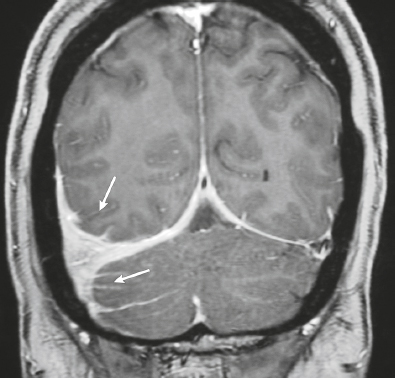
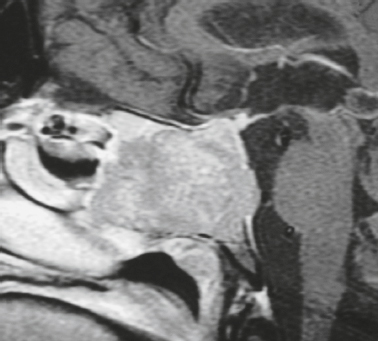
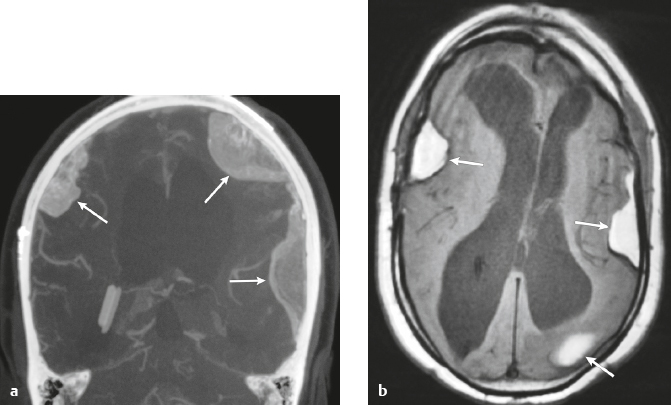
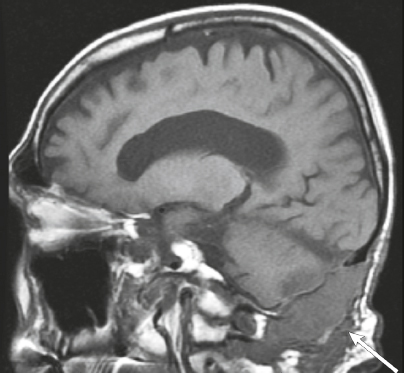
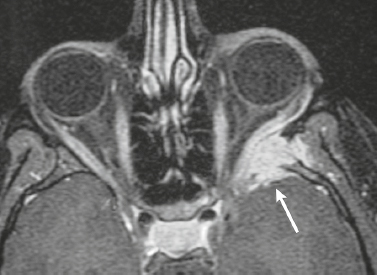
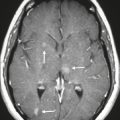
Neurofibromatosis type 1—meningeal dyplasia/ectasia ( Fig. 4.3 ) |
Neurofibromatosis type 1 (NF1) is associated with focal ectasia of intracranial dura, widening of internal auditory canals from dural ectasia, and dural and temporal lobe protrusion into orbit through bony defect (bony hypoplasia of greater sphenoid wing). |
Autosomal dominant disorder (1/2,500 births) representing the most common type of neurocutaneous syndrome, associated with neoplasms of central and peripheral nervous system and skin. Also associated with meningeal and skull dysplasias. |
Neoplastic |
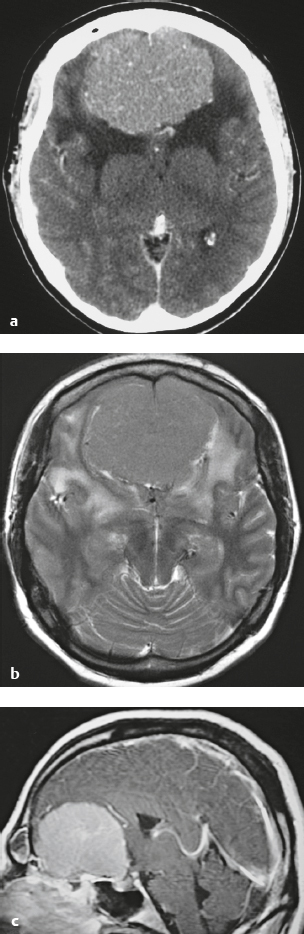
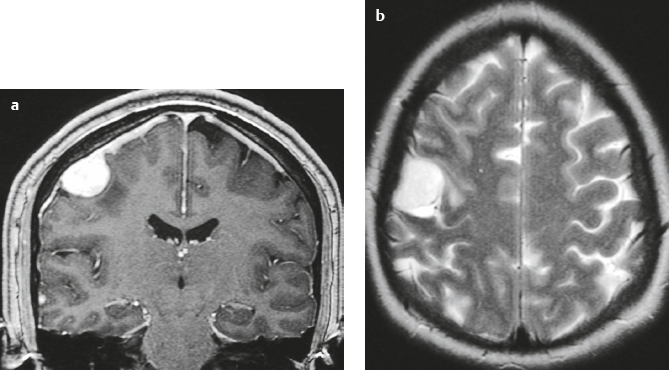
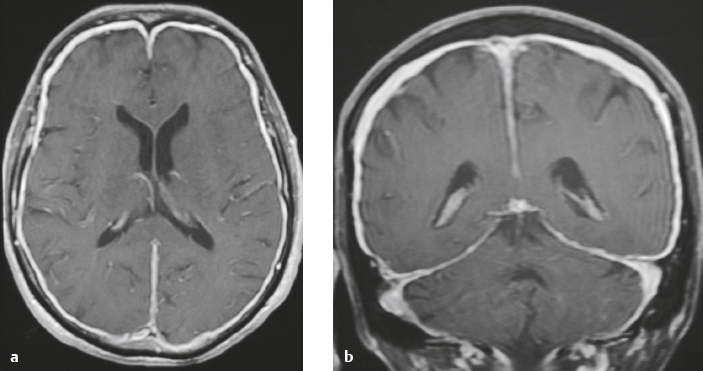
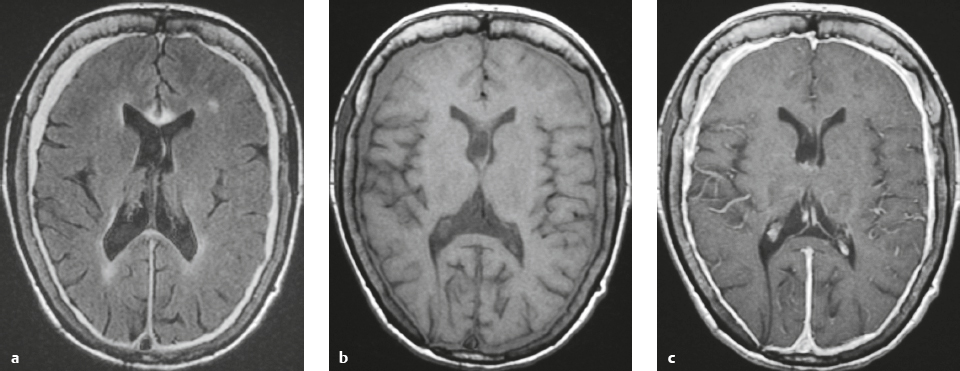
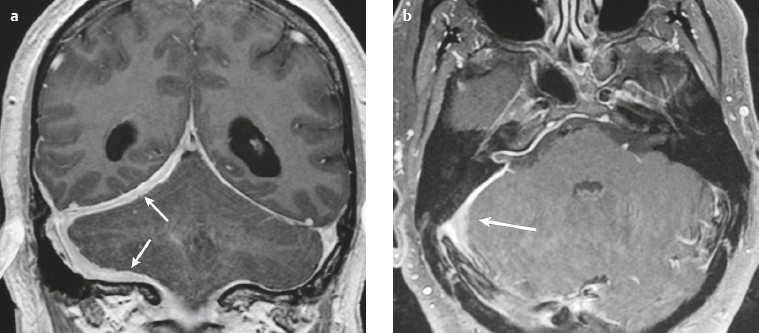
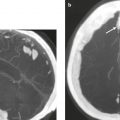
Meningioma ( Fig. 4.4 and Fig. 4.5 ) |
Extra-axial dura-based lesions that are well circumscribed. Locations: supra- >infratentorial, parasagittal > convexity > sphenoid ridge > parasellar > posterior fossa >optic nerve sheath > intraventricular.
MRI: Dura-based tumors with intermediate signal on T1-weighted imaging, intermediate-slightly high signal on T2-weighted imaging, usually prominent gadolinium contrast enhancement often with a dural tail secondary to vasocongestion and interstitial dural edema, ± calcifications. Intratumoral hemorrhage and cystic or necrotic foci can occur in 15% of cases. Can result in compression of adjacent brain parenchyma, encasement of arteries, and compression of dural venous sinuses, rarely invasive/malignant types. Diffusion-weighted imaging/diffusion tensor imaging: ADC values vary among the different subtypes of meningioma. Some tumors can show restricted diffusion, although these findings can be seen with both benign and atypical tumors.
Magnetic resonance spectroscopy: Can show elevated alanine (1.5 ppm), lactate, choline, and glutamine/glutamate levels, and reduced N-acetylaspartate (NAA).
CT: Tumors have intermediate attenuation with or without calcifications, with or without hyperostosis, and usually show prominent contrast enhancement. |
The most common extra-axial tumor, meningioma accounts for up to 26% of primary intracranial tumors. Annual incidence is 6 per 100,000, and it typically occurs in adults (> 40 years old) and in women > in men. Composed of neoplastic meningothelial (arachnoidal or arachnoid cap) cells. Meningiomas are usually solitary and sporadic, but can also occur as multiple lesions in patients with neurofibromatosis type 2. Eighty percent of meningiomas are benign (WHO grade I), although 15% have atypical features (WHO grade II) and ~ 5% have anaplastic histologic features (WHO grade III). Can occur secondary to radiation treatment, with latencies ranging from 19 to 35 years.
Classified into different subtypes, such as meningothelial, fibrous (fibroblastic), transitional (mixed), psammomatous, angiomatous, atypical, and anaplastic. Meningothelial, fibrous, and transitional meningiomas are the most common intracranial types. Usually show immunoreactivity to epithelial membrane antigen (EMA) and vimentin. Secretory meningiomas are typically immunoreactive to CEA. Associated with cytogenetic findings of deletion of chromosome 22. Mutations in the NF2 tumor suppressor gene on chromosome 22 have been found in 60% of sporadic meningiomas. |
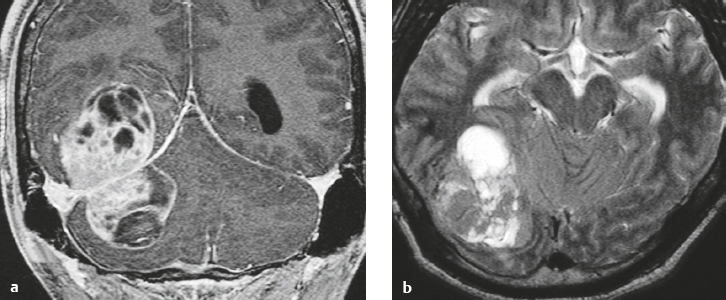
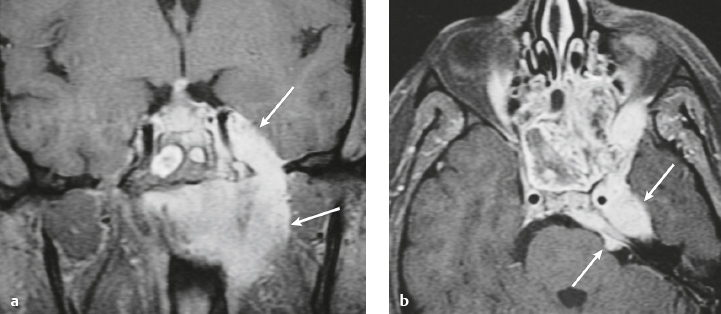
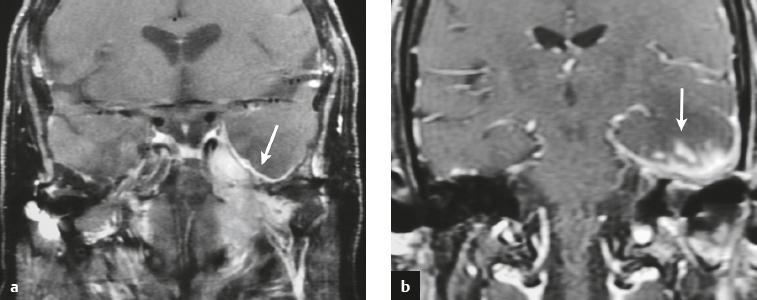
Hemangiopericytoma ( Fig. 4.6 ) |
MRI: Solitary dura-based tumors ranging from 2 to 7 cm in diameter that have low-intermediate signal on T1-weighted imaging, intermediate-slightly high signal on T2-weighted imaging, and usually prominent gadolinium contrast enhancement often with a dural tail, ± calcifications, ± erosion of adjacent bone. Intratumoral hemorrhage and cystic or necrotic foci can occur in 30% of cases. MinADC with hemangiopericytoma is often higher than with most meningiomas (1.1 × 10–3 for hemangiopericytoma versus 0.88 × 10–3 for meningioma).
Magnetic resonance spectroscopy: Relative ratios of myo-inositol (3.56 ppm), glucose, and glutathione with respect to glutamate are higher in hemangiopericytomas than in meningiomas. Absent or low alanine peak for hemangiopericytomas compared with meningiomas.
CT: Tumors are extra-axial mass lesions, often well circumscribed, and have intermediate attenuation with or without calcifications and usually show prominent contrast enhancement. |
Rare (WHO grade II) neoplasms, which account for 0.4% of primary intracranial tumors and are 50 times less frequent than meningiomas. Tumors are composed of closely packed cells with scant cytoplasm and round, ovoid, or elongated nuclei with moderately dense chromatin. Numerous slitlike vascular channels are seen in these tumors that are lined by flattened endothelial cells, ± zones of necrosis. Immunoreactive to vimentin (85%), factor XIIIa (80–100%), and variably to Leu-7 and CD34. Associated with abnormalities involving chromosome 12. Typically occur in young adults (mean age = 43 years) and in males > in females. Sometimes referred to as angioblastic meningioma or meningeal hemangiopericytoma, they arise from vascular cells—pericytes. Recur and metastasize more frequently than meningiomas. |
Solitary fibrous tumor |
MRI: Tumors often have low to intermediate signal on T1-weighted imaging, FLAIR, and proton density-weighted imaging; low, intermediate, and/or slightly high signal on T2-weighted imaging, and heterogeneous slightly high to high signal on fat-suppressed T2-weighted imaging, ± flow voids. After gadolinium contrast administration, solitary fibrous tumors can show prominent, slightly heterogeneous enhancement.
Magnetic resonance spectroscopy: Can show elevated lipid, lactate and myo-inositol levels.
CT: Intermediate to slightly high attenuation, ± calcifications, ± erosion of adjacent bone. |
Rare, benign, spindle-cell mesenchymal neoplasms that occur in a wide range of anatomic sites, such as the pleura, liver, skin, orbits, paranasal sinuses, intracranial dura, and ventricles. Solitary fibrous tumors typically show a hemangiopericytoma-like branching vascular pattern. Mitotic activity is typically low and rarely exceeds 3 per 10 high-power fields. Patient ages range from 20 to 77 years (median age = 50–60 years). |
Epstein-Barr virus– associated smooth muscle tumors |
MRI: Tumors often have low to intermediate signal on T1-weighted imaging and proton density-weighted imaging; low, intermediate, and/or slightly high signal on T2-weighted imaging; and heterogeneous slightly high to high signal on fat-suppressed T2-weighted imaging. After gadolinium contrast administration, SFTs can show prominent slightly heterogeneous enhancement.
CT: Tumors have intermediate attenuation, ± calcifications, ± erosion of adjacent bone. |
In immunocompromised patients, Epstein-Barr virus (EBV) can cause development of smooth muscle tumors (such as leiomyoma and leiomyosarcoma) from mesenchymal cells in the dura or intracranial blood vessels. Tumors contain neoplastic spindle cells, with leiomyosarcomas having high mitotic activity. Immunoreactivity to myogenin, actin, and desmin. |
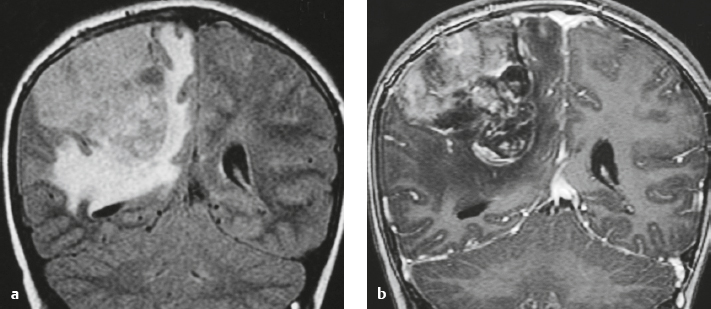
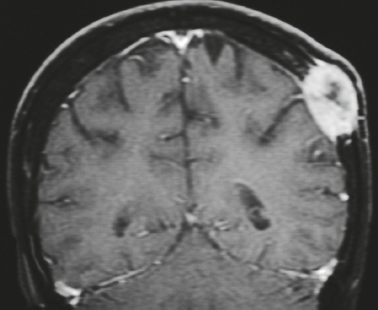
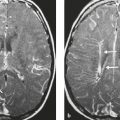
Malignant meningioma ( Fig. 4.7 ) |
MRI: Dura-based tumors with intermediate signal on T1-weighted imaging and intermediate-slightly high signal on T2-weighted imaging, usually with prominent gadolinium contrast enhancement, ± calcifications. Malignant meningiomas are often large and may have irregular margins, with brain invasion and peritumoral edema.
Diffusion-weighted imaging/diffusion tensor imaging: ADC values vary among the different subtypes of meningioma. Some tumors can show restricted diffusion, although these findings can be seen with both benign, atypical, and malignant tumors.
Magnetic resonance spectroscopy (MRS): Can show elevated alanine (1.5 ppm), lactate, choline, and glutamine/glutamate peaks, and reduced N-acetylaspartate (NAA). MRS cannot reliably differentiate benign from malignant meningiomas.
CT: Tumors have intermediate attenuation with or without calcifications, with or without hyperostosis, and usually show prominent contrast enhancement. |
Eighty percent of meningiomas are benign (WHO grade I), although 15% have atypical features (WHO grade II) and ~ 5% have anaplastic/malignant histologic features (WHO grade III). Atypical and anaplastic/malignant meningiomas are associated with 5-year recurrence rates of 40% and 50–80%, respectively. |
Anaplastic hemangiopericytoma |
MRI: Solitary, lobulated, dura-based tumors that have low-intermediate signal on T1-weighted imaging, mixed intermediate, slightly high, and high signal on T2-weighted imaging, and usually prominent heterogeneous gadolinium contrast enhancement. Intratumoral hemorrhage and cystic or necrotic foci are often present, ± dural tail, ± calcifications, ± bone destruction, ± peritumoral edema.
CT: Tumors have intermediate attenuation with or without calcifications and usually show prominent contrast enhancement. |
Anaplastic hemangioperictyomas (WHO grade III) have high degrees of nuclear atypia, with mitotic activities of greater than five mitoses per ten high-power fields. Ki-67 activity >15%. Recurrence and metastases are more frequent than for WHO grade II hemangiopericytomas. |
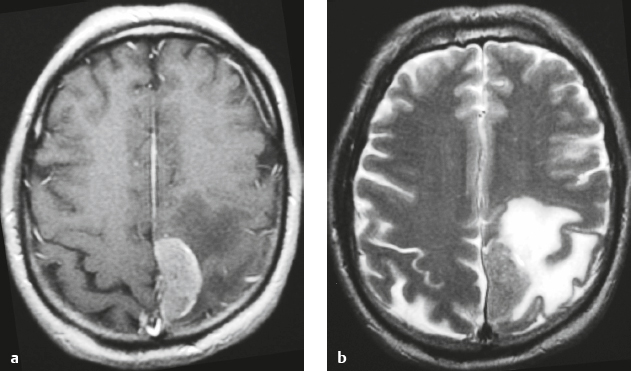
Metastatic tumor ( Fig. 4.8 ) |
MRI: Circumscribed spheroid lesion in dura, leptomeninges, and/or choroid plexus. Often has low-intermediate signal on T1-weighted imaging and intermediate-high signal on T2-weighted imaging, ± hemorrhage, calcifications, and cysts. Variable gadolinium contrast enhancement.
CT: Lesions usually have low-intermediate attenuation; ± hemorrhage, calcifications, and cysts; variable contrast enhancement; ± bone destruction, ± compression of neural tissue or vessels. Leptomeningeal tumor often best seen on postcontrast images. |
Represent ~ 33% of intracranial tumors, usually from extracranial primary neoplasm in adults > 40 years old. Primary tumor source: lung > breast > GI > GU > melanoma. Can occur as single or multiple well-circumscribed or poorly defined lesions involving the skull, dura, leptomeninges, and/or choroid plexus. Metastatic tumor may cause variable destructive or infiltrative changes in single or multiple sites of involvement. |
Lymphoma ( Fig. 4.9 ) |
MRI: Single or multiple well-circumscribed or poorly defined lesions involving the skull, dura, and/or leptomeninges, with low-intermediate signal on T1-weighted imaging and intermediate-high signal on T2-weighted imaging, usually + gadolinium contrast enhancement, ± bone destruction. Leptomeningeal tumor is often best seen on postcontrast images.
CT: CNS lymphoma can have intermediate attenuation or can be hyperdense related to a high nuclear/cytoplasm ratio. FDG PET/CT can show elevated uptake in PCNSL, and in immunocompromised patients it can be used to distinguish lymphoma from toxoplasmosis brain lesions, which have decreased FDG uptake. Usually + contrast enhancement, ±bone destruction. Dural and/or leptomeningeal tumor is usually best seen on postcontrast images. |
Primary CNS lymphoma more common than secondary, usually in adults > 40 years old. Accounts for 5% of primary brain tumors. Incidence currently ranges from 0.8 to 1.5% of primary intracranial tumors. Prior elevated incidence of 6% in patients with AIDS has been reduced with effective antiretroviral therapy. B-cell lymphoma is more common than T-cell lymphoma. MRI features of primary and secondary lymphoma of brain overlap. Intracranial lymphoma can involve the dura and/or leptomeninges in secondary lymphoma > primary lymphoma. Extra-axial lymphoma may cause variable destructive or infiltrative changes in single or multiple sites of involvement. |
Leukemia |
MRI: Single or multiple well-circumscribed or poorly defined lesions involving the skull, dura, and/or leptomeninges with low-intermediate signal on T1-weighted imaging and intermediate-high signal on T2-weighted imaging, usually + gadolinium contrast enhancement, ± bone destruction. Leptomeningeal tumor often best seen on postcontrast images.
CT: Dural and/or leptomeningeal tumor often best seen on postcontrast images. |
Leukemias are neoplastic proliferations of hematopoietic cells. Myeloid sarcomas (also referred to as chloromas or granulocytic sarcomas) are focal tumors composed of myeloblasts and neoplastic granulocyte precursor cells and occur in 2% of patients with acute myelogenous leukemia. These lesions can involve the dura, leptomeninges, and brain. Intracranial lesions can be solitary or multiple. |
Melanocytic neoplasms |
MRI: Lesions have low-intermediate or high signal (secondary to increased melanin) on T1-weighted imaging, intermediate-slightly high and/or low signal on T2-weighted imaging and FLAIR, and gadolinium contrast enhancement.
CT: May show subtle hyperdensity secondary to increased melanin. |
Melanocytoma is a rare benign tumor derived from melanocytes present within the leptomeninges. Tumors contain aggregates of cells with melanin in the cytoplasm. Immunoreactive to HMB-45, melan-A, and S-100. Treatment is surgery with or without radiation treatment. Local recurrence rate is 20%. Localized primary dural melanoma with high mitotic activity, hemorrhage, and/or necrosis can have imaging features that overlap those of melanocytoma. |
Malignant mesenchymal non-meningothelial tumors ( Fig. 4.10 ) |
MRI and CT findings of these lesions are dependent on their histologic features. Malignant tumors may be associated with invasion of adjacent brain, bone, and/or leptomeninges. |
Malignant mesenchymal tumors (WHO grades III and IV) can rarely occur as solitary lesions involving the meninges and skull. Lesions include malignant fibrous histiocytoma, fibrosarcoma, rhabdomyosarcoma, leiomyosarcoma, liposarcoma, chondrosarcoma, osteosarcoma, Ewing′s sarcoma, and angiosarcoma. |
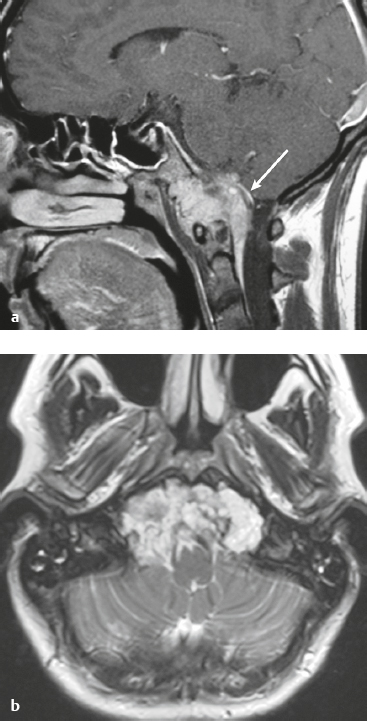
Skull-base tumors ( Fig. 4.11, Fig. 4.12, and Fig. 4.13 ) |
MRI: Destructive bone tumors extending intracranially can cause focal and/or diffuse dural thickening with gadolinium contrast enhancement. |
Myeloma, primary bone tumors (chordoma, chondrosarcoma, osteogenic sarcoma, Ewing′s sarcoma), and neoplasms from the sinuses and nasopharynx (squamous cell carcinoma, nasopharyngeal carcinoma, adenoid cystic carcinoma, and esthesioneuroblastoma) can invade the dura. |
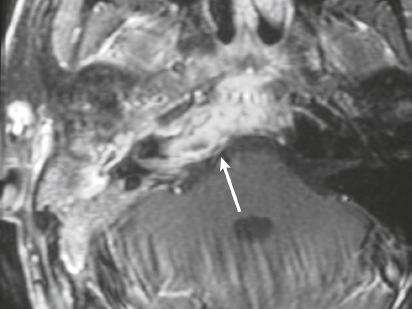
Perineural tumor spread from the sinuses and nasopharynx ( Fig. 4.14 ) |
MRI: Neoplasms can extend from below the skull base into the foramina to involve the dura, leptomeninges, brainstem, and/or brain. Typically show gadolinium contrast enhancement. |
Malignant tumors like sinonasal squamous cell carcinoma, nasopharyngeal carcinoma, adenoid cystic carcinoma, and esthesioneuroblastoma can extend intracranially along the cranial nerves to involve the dura and/or leptomeninges. |
Vascular Lesions |
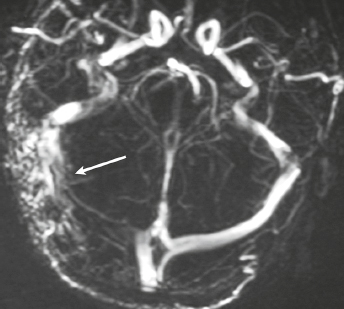
Dural arteriovenous malformation (AVM) ( Fig. 4.15 ) |
Dural AVMs contain multiple, tortuous, tubular vessels. The venous portions often show contrast enhancement. MRA and CTA can show patent portions of the vascular malformation and areas of venous sinus occlusion or recanalization. Usually not associated with mass effect unless there is recent hemorrhage or venous occlusion. |
Dural AVMs are usually acquired lesions resulting from thrombosis or occlusion of an intracranial venous sinus with subsequent recanalization resulting in direct arterial to venous sinus communications. Occur in transverse, sigmoid venous sinuses > cavernous sinus > straight, superior sagittal sinuses. |
Traumatic/Postsurgical Abnormalities |
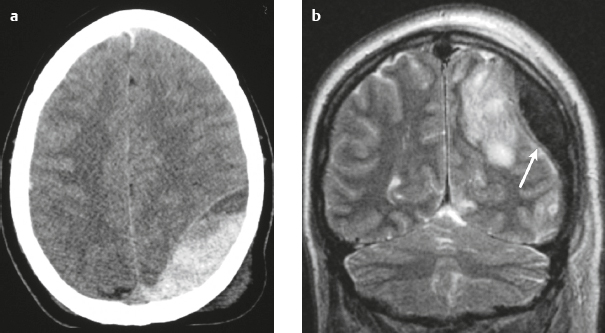
Epidural hematoma ( Fig. 4.16 ) |
The CT attenuation and MRI signal of the hematoma depend on its age, size, hematocrit, and oxygen tension.
MRI: Biconvex extra-axial hematoma located between the skull and dura. Displaced dura has low signal on T2-weighted imaging, ± edema (high signal on T2-weighted imaging involving the displaced brain parenchyma), ± subfalcine or uncal herniation. Hyperacute: Intermediate signal on T1-weighted imaging, intermediate-high signal on T2-weighted imaging.
Acute: Low-intermediate signal on T1-weighted imaging, high signal on T2-weighted imaging.
Early Subacute: High signal on T1-weighted imaging and low signal on T2-weighted imaging.
CT: Biconvex extra-axial hematoma located between the skull and dura. Displaced dura has high attenuation, ± low-attenuation edema involving the displaced brain parenchyma, ± subfalcine or uncal herniation. |
Epidural hematomas usually result from trauma/tearing of an epidural artery (often the middle meningeal artery) or dural venous sinus, ± skull fracture. Epidural hematomas do not cross cranial sutures. |
Hemorrhagic Lesion |
Subdural hematoma ( Fig. 4.17 ) Hyperacute hematoma Acute hematoma Subacute hematoma Chronic hematoma |
Crescentic extra-axial hematoma located in the potential space between the inner margin of the dura and outer margin of the arachnoid membrane, ± edema (low attenuation on CT and high signal on T2-weighted imaging) involving the displaced brain parenchyma, ± subfalcine or uncal herniation. The CT attenuation and MRI signal of the hematoma depend on its age, size, hematocrit, and oxygen tension.
Hyperacute hematoma
MRI: Intermediate signal on T1-weighted imaging and intermediate-high signal on T2-weighted imaging.
CT: Can have high or mixed high, intermediate, and/or low attenuation.
Acute hematoma
MRI: Low-intermediate signal on T1-weighted imaging and low signal on T2-weighted imaging.
CT: Can have high or mixed high, intermediate, and/or low attenuation.
Subacute hematoma MRI: High signal on T1-weighted imaging and T2-weighted imaging.
CT: Can have intermediate attenuation (isodense to brain) and/or low-intermediate attenuation.
Chronic hematoma
MRI: Variable, often low-intermediate signal on T1-weighted imaging and high signal on T2-weighted imaging, ± gadolinium contrast enhancement of collection and organizing neomembrane. Mixed MRI signal can result if rebleeding occurs into chronic collection.
CT: Usually has low attenuation (hypodense to brain). |
Subdural hematomas usually result from trauma/stretching/tearing of cortical veins where they enter the subdural space to drain into dural venous sinuses, ± skull fracture. Subdural hematomas do cross sites of cranial sutures. |
Ossified hematoma ( Fig. 4.18 ) |
MRI: Thin or irregular peripheral zones with low signal from calcifications; peripheral and/or central ossification surrounding collections with variable low, intermediate, and/or high signal on T1- and T2-weighted imaging, ± gadolinium contrast enhancement of collection and organizing neomembrane. Mixed MRI signal can result if rebleeding occurs into chronic collection.
CT: Usually has dense thin or irregular peripheral calcifications, and/or peripheral and central ossification surrounding collections with variable low, intermediate, and/or high attenuation. |
Chronic subdural or epidural hematomas can rarely become calcified or ossified, and are referred to as armored brain. Surgical removal of symptomatic lesions can be difficult because of dense adhesion to brain surface and/or dura. |
Postsurgical dural fibrosis ( Fig. 4.19 and Fig. 4.20 ) |
MRI: Diffuse thickened smooth gadolinium contrast enhancement of dura. The thickened dura can have intermediate signal on T1-weighted imaging and slightly high to high signal on T2-weighted imaging and FLAIR. |
Thickened smooth pachymeningeal (dural) contrast enhancement can occur along the endocranial surface and/or dural reflections after surgery and can persist for years. Typically not associated with clinical signs. |
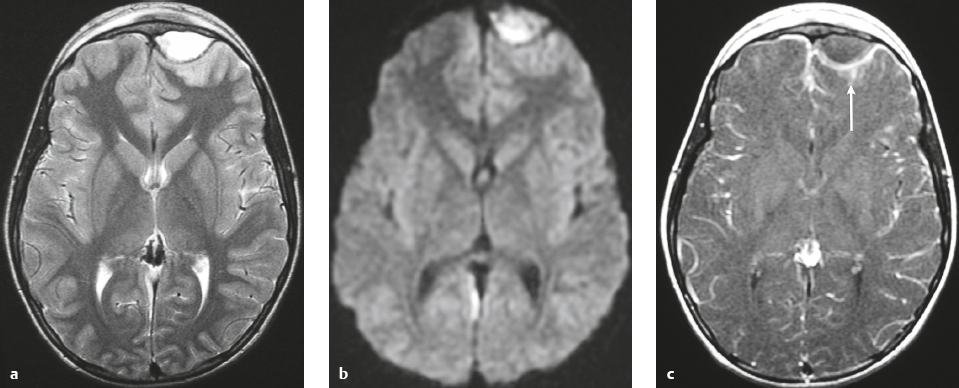
Intracranial hypotension ( Fig. 4.21 ) |
MRI: Diffuse, thickened, smooth gadolinium contrast enhancement of dura; subdural fluid collections/hygromas; pituitary hyperemia; engorgement of intracranial veins and venous sinuses; “sagging“of the brainstem, with tonsillar descent below the foramen magnum; flattening of the ventral surface of the pons; decreased CSF in the interpeduncular and ambient cisterns; downward positioning of the optic chiasm and mamillary bodies with decreased mamillary body– pontine distances, which often measure approximately 4.5 mm (normal distance > 6–7 mm); downward positioning of the iter of the third ventricle below the incisural line; and decreased mean pontomesencephalic angle, which often measures approximately 41° (normal = 65°). The pontomesencephalic angle is the angle between a line drawn anteriorly from the junction of the midbrain and pons; and a line along the anterior margin of the midbrain.
Mild cases of postoperative intracranial hypotension can have findings similar to those in spontaneous intracranial hypotension.
In cases of rapid intraoperative and postoperative CSF loss, abnormal high signal on T2-weighted imaging in the bilateral thalami and basal ganglia can occur in association with impaired responsiveness/neurologic injury after surgery. |
Can occur spontaneously with CSF leak, usually from the spinal canal, related to tears of the dura or nerve root sheaths. Associated with orthostatic headaches relieved by having the patient lie supine. Intracranial hypotension can also occur after lumbar puncture, myelogram, interventional procedure, or surgery. The exact site of CSF loss may not be clearly defined. Incidence is 5 per 100,000 cases per year, peak age of incidence is 40 years, and occurs in women two times more frequently than in men. |
Postsurgical meningocele ( Fig. 4.22 ) |
CSF-filled collection contiguous with the subarachnoid space protruding through a surgical bony defect. |
Usually are not clinically significant unless it becomes large or infected. |
Inflammatory |
Pachymeningitis from infection ( Fig. 4.23 ) |
MRI: Usually shows gadolinium contrast enhancement of thickened dura, ± adjacent leptomeningeal gadolinium contrast enhancement, ± brain abscess and ventriculitis, ± skull osteomyelitis, ± sinus pyocele. Thickened dura can have intermediate signal on T1-weighted imaging and slightly high to high signal on FLAIR and T2-weighted imaging.
CT: Zones of abnormal decreased attenuation, focal sites of bone destruction, ± complications, including subgaleal empyema, epidural empyema, subdural empyema, cerebritis, intra-axial abscess, and venous sinus thrombosis. |
Infection of the dura can result from surgery, trauma, hematogenous dissemination from another source of infection, or direct extension of infection from an adjacent site, such as the brain, leptomeninges, skull, orbits paranasal sinuses, and nasopharynx. Intracranial extension of skull base infection typically involves the dura first, followed by the leptomeninges and brain. |
Epidural/subdural abscess/empyema ( Fig. 4.24 and Fig. 4.25 ) |
MRI: Epidural or subdural collections with low signal on T1-weighted imaging, high signal on T2-weighted imaging, and thin or irregular peripheral zones of gadolinium contrast enhancement.
CT: Epidural or subdural collections with low attenuation and peripheral zones of contrast enhancement |
Often results from complications related to sinusitis (usually frontal), meningitis, otitis media, ventricular shunts, or surgery. Can be associated with venous sinus thrombosis and venous cerebral or cerebellar infarctions, cerebritis, brain abscess; mortality 30%. |
Langerhans’ cell histiocytosis ( Fig. 4.26 ) |
MRI: Fusiform or lobulated lesion with intermediate signal on T1- and T2-weighted imaging involving hypothalamus/pituitary stalk, skull, dura, and rarely the leptomeninges. Lesions in skull, dura, pituitary stalk, and leptomeninges usually show gadolinium contrast enhancement.
CT: Intraosseous lesions usually have low-intermediate attenuation and are typically associated with localized bone destruction, + contrast enhancement of the adjacent dura, and rarely the leptomeninges. Thickening of the pituitary stalk may be seen. |
Disorder of reticuloendothelial system in which bone marrow–derived dendritic Langerhans’ cells infiltrate various organs as focal lesions or in diffuse patterns. Langerhans’ cells have eccentrically located ovoid or convoluted nuclei within pale to eosinophilic cytoplasm. Lesions often consist of Langerhans’ cells, macrophages, plasma cells, and eosinophils. Lesions are immunoreactive to S-100, CD1a, CD-207, HLA-DR, and β2-microglobulin. LCH has been associated with BRAF or MAP2K1 mutations. Prevalence of 2 per 100,000 children less than 15 years old; only a third of lesions occur in adults. Localized lesions (eosinophilic granuloma) can be single or multiple in the skull with involvement of adjacent dura, usually at the skull base. Can also involve the dura without adjacent osseous disease. Intradural lesions occur at pituitary stalk/hypothalamus and can present with diabetes insipidus. Lesions rarely occur in brain tissue (< 4% of patients with Langerhans’ cell histiocytosis). Occur in patients with median age = 10 years, average = 13.5 years. Peak incidence is in patients between 5 and 10 years old; 80 to 85% occur in patients less than 30 years old. |
Erdheim-Chester disease |
MRI: Lesions in the CNS can occur in the cerebrum, hypothalamus, cerebellum, and choroid plexus, as well as in spinal or cranial dura. Single or multiple dura-based lesions can be seen with low to intermediate signal on T1-weighted imaging and proton density-weighted imaging and with low, intermediate, and/or slightly high signal on T2-weighted imaging. After gadolinium contrast administration, lesions usually show enhancement. Prolonged gadolinium contrast enhancement may occur from contrast retention by histiocytes.
CT: Intermediate attenuation, ± osteosclerosis of craniofacial bones. |
Rare multisystem non-Langerhans’ cell histiocytic disorder of unknown etiology that usually affects adults. Collections are formed of foamy lipid-laden histiocytes with small bland nuclei, Touton-like giant cells, multinucleated giant cells, fibrosis/dense collagen, chronic inflammatory cells (lymphocytes and histiocytes), and occasional scattered eosinophils. Immunoreactive to the histiocytic antigen CD68 and show variable or no reactivity to S-100. Unlike Langerhans’ cell histiocytosis, Erdheim-Chester disease lesions lack immunoreactivity to CD1a. Can involve the musculoskeletal, pulmonary, cardiac, gastrointestinal, and central nervous systems. Age range is 7 to 84 years. Most common in fourth to seventh decades. Prognosis depends on the extent and location of disease. Treatment includes surgical debulking, prednisone, cyclosporine, vincristine, vinblastine, cyclophosphamide, and/or doxorubicin. Radiation treatment may be useful for intracranial lesions in the brain parenchyma and other locations. Immunotherapy with agents like interferon-a2a has also been used. Multisystem disease can progress to death from respiratory distress, pulmonary fibrosis, and renal and/or heart failure. Thirty-seven percent of patients had died after a mean follow-up interval of 32 months. |
Rosai-Dorfman disease |
MRI: Single or multiple dural lesions can be seen with intermediate signal on T1-weighted imaging and low-intermediate signal on T2-weighted imaging. Low signal centrally on T2-weighted imaging may be secondary to histiocytic release of free radicals. After gadolinium contrast administration, lesions usually show enhancement.
CT: Dura-based lesion with intermediate attenuation, ± erosion of adjacent bone. |
Rare benign histiocytosis in which collections of lymphoplasmacytic cells and histiocytes occur in fibrous stroma within various tissues, such as lymph nodes, bone, orbits, nasal cavity, and intracranial dura. Immunoreactive to S-100 protein and CD68 (macrophages) and lacks immunoreactivity to CD1a (Langerhans’ cell marker). Occurs in children and young adults (peak age between 30 and 40 years). |
Sarcoidosis ( Fig. 4.27 ) |
MRI: Smooth and/or nodular gadolinium contrast enhancement in the leptomeninges and/or dura are often seen. Lesions in the brain can show poorly marginated intra-axial zones with low-intermediate signal on T1-weighted imaging and slightly high to high signal on T2-weighted imaging and FLAIR, usually with gadolinium contrast enhancement, + localized mass effect and peripheral edema.
CT: Smooth and/or nodular contrast enhancement in the leptomeninges and/or dura can be seen. Intra-axial lesions usually have poorly defined margins, low-intermediate attenuation, usually with contrast enhancement, + localized mass effect and peripheral edema. |
Sarcoidosis is a multisystem noncaseating granulomatous disease of uncertain cause that can involve the CNS in 5 to 15% of cases. If untreated, it is associated with severe neurologic deficits, such as encephalopathy, cranial neuropathies, and myelopathy. Diagnosis of neurosarcoid may be difficult when the neurologic complications precede other systemic manifestations in the lungs, lymph nodes, skin, bone, and/or eyes. |
Granulomatosis with polyangiitis |
MRI: Poorly defined zones of soft tissue thickening with low-intermediate signal on T1-weighted imaging, slightly high to high signal on T2-weighted imaging, and gadolinium contrast enhancement within the nasal cavity, paranasal sinuses, infratemporal fossa, and external auditory canal, ± bone invasion and destruction, ± extension into the skull base. Intracranially, involvement of the dura, leptomeninges, brain, or venous sinuses can occur.
CT: Zones with soft tissue attenuation, ± bone destruction. |
Multisystem disease with necrotizing granulomas in the respiratory tract, focal necrotizing angiitis of small arteries and veins of various tissues, and glomerulonephritis. Often involves the upper respiratory tract, lungs, and kidneys, and can involve the paranasal sinuses, orbits, and temporal bone. Peak incidence is in the fifth decade, and annual incidence is 8 per million. Antibodies to neutrophil cytoplasmic antigens (ANCA) occur in 80–90% of patients and play a role in pathogenesis. |
Inflammatory pseudotumor ( Fig. 4.28 ) |
MRI: Smooth and/or nodular gadolinium contrast enhancement in the dura and/or leptomeninges, ± extension from the skull base, infratemporal soft tissue, and/or orbits. Lesions in the brain can appear as poorly marginated intra-axial zones with low-intermediate signal on T1-weighted imaging and slightly high to high signal on T2-weighted imaging and FLAIR, usually with gadolinium contrast enhancement, + localized mass effect and peripheral edema.
CT: Smooth and/or nodular contrast enhancement in the leptomeninges and/or dura can be seen. Intra-axial lesions usually have poorly defined margins and low-intermediate attenuation and usually show contrast enhancement, + localized mass effect and peripheral edema. |
Chronic inflammatory disorder of unknown etiology with lesions composed of spindle cells, lymphocytes, and plasma cells. Lesions can occur in many locations, most commonly in the orbits and lungs. Rarely occur in the skull base, dura, and brain. Usually occur in children and young adults. Can be asymptomatic or associated with localized mass effect as well as systemic signs, such as weight loss and fever. Treatment of orbital inflammatory pseudotumor is typically with steroid medication. Surgery is often performed for lesions in other locations. |
Idiopathic hypertrophic pachymeningitis |
MRI: Thickened linear pattern of gadolinium contrast enhancement involving the dura. ± Lesions in the brain, which can appear as poorly marginated intra-axial zones with low-intermediate signal on T1-weighted imaging and slightly-high to high signal on T2-weighted imaging and FLAIR, usually with gadolinium contrast enhancement, + localized mass effect and peripheral edema.
CT: Thickened linear pattern of contrast enhancement involving the intracranial dura can be seen. ± Intra-axial lesions in the brain with decreased attenuation and poorly defined margins. |
Clinical disorder with diffuse and/or localized thickening and gadolinium contrast enhancement of dura without history of associated trauma, neoplasms, infection, or inflammatory diseases, such as rheumatoid arthritis, granulomatosis with polyangiitis, IgG4 disease, etc. Occurs more commonly in men than in women, age 39 to 88 years (mean age = 55 years). Clinical findings include headache, loss of vision, and diplopia. Dural biopsies show collections of small, mature lymphocytes, epithelioid histiocytes, and plasma cells, as well as absence of microorganisms, vasculitis, and neoplastic cells. Clinical symptoms like headaches and vision loss can progress. For these patients, treatment has consisted of steroids and other immunosuppressive drugs. |