Malignant neoplasms |
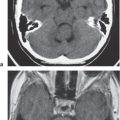
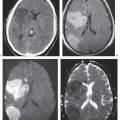
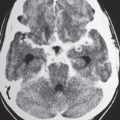
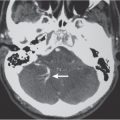
Metastatic tumor
Fig. 3.18 |
Single or multiple well-circumscribed or poorly defined lesions involving the skull, dura, leptomeninges, brain, and/or choroid plexus; often show contrast enhancement, with or without bone destruction, with or without compression of neural tissue or vessels. Leptomeningeal tumor often best seen on postcontrast images. |
May have variable destructive or infiltrative changes involving single or multiple sites of involvement. Primary tumors are usually from outside CNS. |
Myeloma/plasmacytoma |
Multiple (myeloma) or single (plasmacytoma) well-circumscribed or poorly defined lesions involving the skull and dura; low to intermediate attenuation; usually show contrast enhancement, with bone destruction. |
Malignant plasma cell tumor; may have variable destructive or infiltrative changes involving the axial and/or appendicular skeleton. |
Lymphoma |
Single or multiple well-circumscribed or poorly defined lesions involving the skull, dura, and/or leptomeninges; low to intermediate attenuation; may show contrast enhancement, with or without bone destruction. Leptomeningeal tumor often best seen on postcontrast images. |
Extra-axial lymphoma may have variable destructive or infiltrative changes involving single or multiple sites of involvement. |
Leukemia |
Single or multiple well-circumscribed or poorly defined lesions involving the skull, dura, and/or leptomeninges; low to intermediate attenuation; may show contrast enhancement, with or without bone destruction. Leptomeningeal tumor often best seen on postcontrast images. |
Extra-axial lymphoma may have variable destructive or infiltrative changes involving single or multiple sites of involvement. |
Chordoma
Fig. 3.19a–c |
Well-circumscribed, lobulated lesions; low to intermediate attenuation; usually shows contrast enhancement (usually heterogeneous); locally invasive associated with bone erosion/destruction, encasement of vessels and nerves; skull base/clivus common location, usually in the midline. |
Rare, slow-growing tumors at the skull base; detailed anatomical display of extension of chordomas by CT and MRI is important for planning of surgical approaches. |
Chondrosarcoma
Fig. 3.20a–c |
Lobulated lesions, low to intermediate attenuation, with or without matrix mineralization; can show contrast enhancement (often heterogeneous); locally invasive associated with bone erosion/destruction, encasement of vessels and nerves, skull base/petrous/occipital synchondrosis common location, usually off midline. |
Rare, slow-growing, malignant cartilaginous tumors; detailed anatomical display of extension of chondrosarcomas by CT and MRI is important for planning of surgical approaches. |
Osteogenic sarcoma
Fig. 3.21a, b |
Destructive lesions involving the skull base and calvarium; low to intermediate attenuation, usually with matrix mineralization/ossification; often shows contrast enhancement (usually heterogeneous). |
Rare lesions involving the skull base and calvarium; more common than chondrosarcomas and Ewing sarcoma; locally invasive, high metastatic potential. Occurs in children as primary tumors and adults (associated with Paget disease, irradiated bone, chronic osteomyelitis, osteoblastoma, giant cell tumor, and fibrous dysplasia). |
Ewing sarcoma
Fig. 3.22 |
Destructive lesions involving the skull base and calvarium; low to intermediate attenuation; can show contrast enhancement (usually heterogeneous). |
Malignant bone tumors that usually occur between the ages of 5 and 30, males > females; rare lesions involving the skull base; locally invasive, high meta-static potential. |
Sinonasal/nasopharyngeal carcinoma
Fig. 3.23a–c |
Destructive lesions in the nasal cavity, paranasal sinuses, nasopharynx; with or without intracranial extension via bone destruction or perineural spread; intermediate attenuation, can show contrast enhancement; large lesions (with or without necrosis and/or hemorrhage). |
Occurs in adults usually older than age 55 y, men = women; associated with occupational or other exposure to nickel, chromium, mustard gas, radium, and manufacture of wood products. |
Adenoid cystic carcinoma |
Destructive lesions in the paranasal sinuses, nasal cavity, nasopharynx; with or without intracranial extension via bone destruction or perineural spread; intermediate attenuation, variable degrees of contrast enhancement. |
Account for 10% of sinonasal tumors; arise in any location within sinonasal cavities; usually occurs in adults older than age 30 y. |
Esthesioneuroblastoma |
Locally destructive lesions with low to intermediate attenuation; usually shows contrast enhancement. Location: superior nasal cavity, ethmoid air cells with occasional extension into the other paranasal sinuses, orbits, anterior cranial fossa, cavernous sinuses. |
Malignant tumors also referred to as olfactory neuroblastoma arise from olfactory epithelium in the superior nasal cavity. Occur in adolescents and adults, men = women. |
Rhabdomyosarcoma |
Lesions have low to intermediate attenuation with circumscribed and/or poorly defined margins. Areas of hemorrhage may be present. Lesions may have heterogeneous attenuation. Zones of edema may occur in the adjacent soft tissues. Tumors can be associated with destructive changes of adjacent bone; show variable degrees and patterns of contrast enhancement. |
Malignant mesenchymal tumors with rhabdomyoblastic differentiation that occur primarily in soft tissue and only very rarely in bone. Occur most frequently in children. |
Hemangiopericytoma |
Extra-axial mass lesions, often well circumscribed; intermediate attenuation; usually show prominent contrast enhancement (may resemble meningiomas); with or without associated erosive bone changes. |
Rare neoplasms in young adults (males > females) sometimes referred to as angioblastic meningioma or meningeal hemangiopericytoma; arise from vascular cells/pericytes; frequency of metastases > meningiomas. |
Meningioma
Fig. 3.24a, b |
Extra-axial dural-based lesions, well-circumscribed; supratentorial > infratentorial; parasagittal > convexity > sphenoid ridge > parasellar > posterior fossa > optic nerve sheath > intraventricular; intermediate attenuation; typically show prominent contrast enhancement, with or without calcifications, with or without hyperostosis and/or invasion of adjacent skull. |
Most common extra-axial tumors; usually benign neoplasms; typically occur in adults (> 40 y), women > men; multiple meningiomas seen with neurofibromatosis type II; can result in compression of adjacent brain parenchyma, encasement of arteries, and compression of dural venous sinuses; rarely invasive/malignant types. |
Hemangioma
Fig. 3.25 |
Circumscribed or poorly marginated structures (< 4 cm in diameter) in marrow of skull (often frontal bone) with intermediate attenuation; prominent bone trabeculae may be seen; typically show contrast enhancement, with or without widening of diploic compartment. |
Benign skull lesions, adults (> 30 y). |
Ossifying hemangioma
Fig. 3.26a–c |
Zone with low to intermediate attenuation; usually show prominent contrast enhancement. |
Benign lesions within the temporal bone that involve the facial nerve and on CT are usually radiolucent, containing bone spicules. Lesions can be associated with slowly progressive or recurrent facial paralysis. |
Osteoid osteoma
Fig. 3.27 |
Intraosseous circumscribed radiolucent lesion < 1.5 cm in diameter surrounded by bone sclerosis. Lesions often have low to intermediate attenuation centrally; often show contrast enhancement, surrounded by a peripheral rim of increased attenuation from associated bone sclerosis. |
Benign osseous lesion containing a nidus of vascularized osteoid trabeculae surrounded by osteoblastic sclerosis that rarely occurs in the skull. Usually occurs between the ages of 5 and 25 y, males > females. Focal pain and tenderness associated with lesion that is often worse at night, relieved with aspirin. |
Osteoblastoma |
Expansile radiolucent lesion often > 1.5 cm surrounded by bone sclerosis. Lesions can show contrast enhancement. |
Rare benign bone neoplasm (2% of bone tumors) usually occurs between the ages of 6 and 30 y; rarely involves the skull. |
Enchondroma
Fig. 3.28a–c |
Lobulated intramedullary lesion that usually has low to intermediate attenuation and contains areas of chondroid matrix mineralization and fibrous strands. Lesions can show contrast enhancement. |
Benign intramedullary lesions composed of hyaline cartilage; represent <10% of benign bone tumors. Enchondromas can be solitary (88%) or multiple (12%). |
Chondroblastoma |
Tumors often have fine lobular margins and typically have low to intermediate attenuation containing chondroid matrix mineralization (50%); contrast enhancement may be seen. Cortical destruction is uncommon. |
Benign cartilaginous tumors with chondroblast-like cells and areas of chondroid matrix formation that rarely occur in the craniofacial bones. The squamous portion of the temporal bone is the most common location. |
Pituitary adenoma
Fig. 3.29a, b |
Macroadenomas (> 10 mm): Commonly have intermediate attenuation, with or without necrosis, with or without cyst, with or without hemorrhage; usually show contrast enhancement, extension into suprasellar cistern with waist at diaphragma sella, with or without extension into cavernous sinus; occasionally invades skull base. |
Common benign, slow-growing tumors representing <50% of sellar/parasellar neoplasms in adults. Can be associated with endocrine abnormalities related to oversecretion of hormones (prolactin > nonsecretory type > growth hormone > adrenocorticotropic hormone [ACTH]). Prolactinomas: women > men; growth hormone tumors: men = women. |
Paraganglioma/glomus jugulare
Fig. 3.30a–c |
Ovoid or fusiform lesions with low to intermediate attenuation. Lesions can show contrast enhancement; often erode adjacent bone. |
Benign encapsulated neuroendocrine tumors that arise from neural crest cells associated with autonomic ganglia (paraganglia) throughout the body. Lesions, also referred to as chemodectomas, are named according to location (glomus jugulare, tympanicum, vagale). |
Endolymphatic sac cystadenoma |
Extra-axial retrolabyrinthine lesions involving the posterior petrous bone extending into the cerebellopontine angle cistern. Lesions can have low to intermediate attenuation and can show contrast enhancement. May contain blood products. |
Rare solid and/or cystic benign or malignant papillary adenomatous tumors arising from the endolymphatic sac in children and adults. Tumors are slow growing and rarely metastasize; may be sporadic or associated with von Hippel-Lindau disease. |
Other lesions |
Osteoma
Fig. 3.31a–c |
Well-circumscribed lesions involving the skull with high attenuation; typically show no contrast enhancement. |
Benign proliferation of bone located in the skull or paranasal sinuses (frontal > ethmoid > maxillary > sphenoid). |
Epidermoid
Fig. 3.32a–c |
Well-circumscribed, spheroid ectodermal inclusion cystic lesions in the skull associated with chronic bone erosion; low to intermediate attenuation; no contrast enhancement. |
Nonneoplastic lesions filled with desquamated cells and keratinaceous debris involving the skull. |
Dermoid |
Well-circumscribed, spheroid lesions in the skull associated with chronic bone erosion; usually with low attenuation, no contrast enhancement, with or without fluid–fluid or fluid–debris levels. |
Nonneoplastic ectodermal inclusion cystic lesions involving the skull filled with lipid material, cholesterol, desquamated cells, and keratinaceous debris. |
Aneurysmal bone cyst
Fig. 3.33a, b |
Circumscribed extradural vertebral lesion usually involving the posterior elements with or without involvement of the vertebral body; with variable low, intermediate, or high attenuation; with or without lobulations, with or without one or multiple fluid/fluid levels. |
Expansile blood/debris-filled lesions that may be primary or occur secondary to other bone lesions, such as giant cell tumor, fibrous dysplasia, and chondroblastoma. Most occur in patients older than 30 y. These lesions rarely involve the skull. |
Giant cell reparative granuloma |
Lesions are radiolucent and can have heterogeneous low to intermediate attenuation. |
Giant cell reparative granulomas are also referred to as solid aneurysmal bone cysts (ABCs). Histologic appearance resembles brown tumors. |
Arachnoid cyst |
Well-circumscribed, extra-axial lesions with low attenuation similar to CSF; no contrast enhancement. Chronic erosive changes can be seen at the adjacent skull. |
Nonneoplastic acquired, developmental, or congenital extra-axial cysts filled with CSF. Cysts can be small or large, asymptomatic or symptomatic. |
Inflammatory lesions |
Pyogenic osteomyelitis
Fig. 3.34a, b |
Zones of abnormal decreased attenuation, focal sites of bone destruction, with or without complications including subgaleal empyema, epidural empyema, subdural empyema, meningitis, cerebritis, intra-axial abscess, and venous sinus thrombosis. |
Osteomyelitis of the skull can result from surgery, trauma, hematogenous dissemination from another source of infection, or direct extension of infection from an adjacent site, such as the paranasal sinuses. |
Eosinophilic granuloma
Fig. 3.35 |
Single or multiple circumscribed soft tissue lesions in the marrow of the skull associated with focal bony destruction/erosion with extension extracranially, intracranially, or both. Lesions usually have low to intermediate attenuation; can show contrast enhancement, with or without enhancement of the adjacent dura. |
Single lesions commonly seen in males > females younger than age 20 y; proliferation of histiocytes in medullary cavity with localized destruction of bone with extension in adjacent soft tissues. |
|
|
Multiple lesions associated with Letterer-Siwe disease (lymphadenopathy hepatospleno megaly), children younger than 2 y; Hand-Schüller- Christian disease (lymphadenopathy, exophthalmos, diabetes insipidus), children ages 5 to 10 y. |
Sarcoidosis |
Sarcoid lesions within marrow can be multiple or solitary, with or without bone expansion and/or erosions or areas of destruction of the inner and/or outer tables with extension intracranially or into the extracranial soft tissues. Lesions can have circumscribed and/or indistinct margins and usually have low to intermediate attenuation signal; can show variable degrees of contrast enhancement. |
Chronic systemic granulomatous disease of unknown etiology in which noncaseating granulomas occur in various tissues and organs, including bone. |
Paranasal sinus mucocele
Fig. 3.36a, b |
Circumscribed expansile lesion within a paranasal sinus that has variable low, intermediate, and/or high attenuation depending on contents of mucus, inspissated mucus, and protein concentration. |
Lesions occurring from chronic obstruction of a paranasal sinus ostium that results in outward expansion of the osseous margins from remodeling secondary to increased pressure from accumulated secretions from the sinus mucosa. Mucoceles occur most commonly in the frontal sinuses, followed by the ethmoid, maxillary, and sphenoid sinuses. |
Cholesterol granuloma
Fig. 3.37a, b |
Circumscribed lesion measuring between 2 and 4 cm in the marrow of the petrous bone often associated with mild bone expansion. Lesions usually have low attenuation. |
Lesions seen in young and middle-aged adults and occur when there is obstruction of mucosal-lined air cells in the petrous bone. Multiple cycles of hemorrhage and granulomatous reaction result in accumulation of cyst contents with cholesterol granules, chronic inflammatory cells, red blood cells, hemosiderin, fibrous tissue, and debris. |
Acquired |
Aneurysm |
Focal, circumscribed lesion with low to intermediate and/or high attenuation. CTA shows contrast enhancement of nonthrombosed portions of lumens of aneurysms. |
Abnormal dilation of artery secondary to acquired/degenerative cause, connective tissue disease, atherosclerosis, trauma, infection (mycotic), AVM, drugs, and vasculitis. |
Postsurgical pseudomeningocele
Fig. 3.38 |
CSF-filled collection contiguous with the subarachnoid space protruding through a surgical bony defect. Gliotic brain tissue may also accompany the dural protrusion. |
Usually not clinically significant unless it becomes large or infected. |
Paget disease
Fig. 3.39 |
Expansile sclerotic/lytic process involving the skull with mixed intermediate to high attenuation. Irregular/indistinct borders are seen between marrow and inner margins of the outer and inner tables of the skull. |
Usually seen in older adult;, can result in narrowing of neuroforamina with cranial nerve compression, basilar impression, with or without compression of brainstem. |
Fibrous dysplasia
Fig. 3.40 |
Expansile process involving the skull with mixed intermediate and high attenuation, often in a “ground glass” appearance; can show contrast enhancement. |
Usually seen in adolescents and young adults; can result in narrowing of neuroforamina with cranial nerve compression, facial deformities, mono- and polyostotic forms with or without endocrine abnormalities, such as with McCune-Albright syndrome (precocious puberty). |
Hematopoietic disorders |
Enlargement of the diploic space with red marrow hyperplasia and thinning of the inner and outer tables. |
Thickening of diploic space related to erythroid hyperplasia from anemia related to sickle cell disease, thalassemia major, and hereditary spherocytosis. Similar findings of red marrow expansion can be seen with polycythemia rubra. |
Osteopetrosis
Fig. 3.41a, b |
Findings include generalized bone sclerosis, hyper-ostosis resulting in thickening of the skull, as well as narrowing of the foramina and optic canals. |
Heterogeneous group of bone disorders with defective resorption of primary spongiosa and mineralized cartilage from osteoclast dysfunction. Results in failure of conversion of immature woven bone into strong lamellar bone and pathologic fractures. In the severe autosomal recessive form, medullary crowding from immature sclerotic bone can result in anemia, thrombocytopenia, and immune dysfunction leading to death. |
Hyperostosis frontalis
Fig. 3.42 |
Expansion of the medullary portion of the upper frontal bone extending intracranially with well-defined cortical margin of the inner table of the skull. |
Benign bilateral bone overgrowth involving the inner table of the frontal bone; most often seen in elderly women. |
Trauma |
Cephalohematoma
Fig. 3.43a, b |
Hematoma located beneath periosteum of outer table; does not cross suture lines; with or without skull fracture; with or without subdural hematoma. |
Results from birth trauma (complication of forceps delivery); associated with 1% of births. |
Fracture
Fig. 3.44 |
Nondisplaced/nondepressed skull fractures: With or without subgaleal hematoma, with or without epidural hematoma, with or without subdural hematoma, with or without subarachnoid hemorrhage.
Depressed skull fracture: Angulation and internal displacement of fractured skull, with or without subgaleal hematoma, with or without epidural hematoma, with or without subdural hematoma, with or without subarachnoid hemorrhage. |
Traumatic fractures of the skull can involve the calvarium or skull base; significant complications that can result include epidural hematoma, subdural hematoma, subarachnoid hemorrhage, CSF leakage/rhinorrhea, and otorrhea. |
Congenital abnormalities |
Cephaloceles (meningoceles or meningoencephaloceles)
Fig. 3.45a, b |
Defect in skull through which there is herniation of meninges and CSF (meningocele) or meninges, CSF/ventricles, and brain tissue (meningoencephaloceles). |
Congenital malformation involving lack of separation of neuroectoderm from surface ectoderm with resultant localized failure of bone formation. Occipital location most common in Western hemisphere; frontoethmoidal location most common site in Southeast Asians. Other sites are parietal and sphenoid bones. Cephaloceles can also result from trauma or surgery. |
Chiari II malformation/lückenschädel skull
Fig. 3.46a, b |
Multifocal scalloping at the inner table of the skull. |
Also referred to as lacunar skull or craniolacunae, dysplasia of membranous skull/calvarium in Chiari II with multifocal thinning of the inner table from nonossified fibrous bone from abnormal collagen development and ossification. |
Craniosynostosis
Fig. 3.47a, b
Fig. 3.48a–c
Fig. 3.49a, b |
Premature closure; metopic suture results in trigonocephaly (wedge-shaped skull), sagittal suture results in scaphocephaly (long, narrow-shaped skull), coronal or lambdoid suture results in oxycephaly, and unilateral closure of coronal or lambdoid sutures result in plagiocephaly. |
Premature closure of the cranial sutures results in abnormal skull shape. Can be primary from abnormal development or secondary from external forces of intrauterine compression, lack of brain growth, and/or teratogens; 15% are associated with other anomalies; 80% involve one suture. Closure of multiple sutures is often associated with genetic etiology. |
Achondroplasia
Fig. 3.50 |
The calvarium/skull vault is enlarged in association with a small skull base and narrow foramen magnum. Cervicomedullary myelopathy and/or hydrocephalus can result from a narrowed foramen magnum. |
Autosomal dominant mutation (fibroblast growth factor gene 3; 1/10 000 births) in which the mutated gene impairs endochondral bone formation, resulting in decreased longitudinal lengthening of long bones. |
Basiocciput hypoplasia
Fig. 3.51 |
Hypoplasia of the lower clivus results in primary basilar invagination. |
The lower clivus is a portion of the occipital bone (basiocciput) that is composed of four fused sclerotomes. Failure of formation of one or more of these sclerotomes results in a shortened clivus and primary basilar invagination (dens extending > 5 mm above the Chamberlain line). |
Condylus tertius
Fig. 3.52 |
Ossicle seen between the lower portion of a shortened basiocciput and the dens/atlas. |
Condylus tertius or third occipital condyle results from lack of fusion of the lowermost fourth sclerotome (proatlas) with the adjacent portions of the clivus. This third occipital condyle can form a pseudojoint with the anterior arch of C1 and/or dens and can be associated with decreased range of movement. |
Atlanto-occipital assimilation |
Often seen as fusion of the occipital condyle with one or both lateral masses of C1. |
Occurs from failure of segmentation of the occipital condyle and the C1 vertebra. |
Neurofibromatosis type 1 (NF1)
Fig. 3.53a, b |
NF1 associated with focal ectasia of intracranial dura, widening of internal auditory canals from dural ectasia, dural and temporal lobe protrusion into orbit through bony defect (bony hypoplasia of greater sphenoid wing), bone malformation, or erosion from plexiform neurofibromas. |
Autosomal dominant disorder (1/2500 births) representing the most common type of neurocutaneous syndromes; associated with neoplasms of central and peripheral nervous systems and skin. Also associated with meningeal and skull dysplasias. |