5 Management of Complications of Portal Hypertension: Special Considerations
Rex M. Pillai, Mark J. Sands, and Baljendra S. Kapoor
5.1 Introduction
The role of interventional radiology in managing the complications of portal hypertension continues to evolve. The early days of percutaneous intervention were primarily diagnostic; trans-hepatic portography and arterioportography were performed to determine the presence and etiology of portal hypertension and to help with surgical planning. In the current era of noninvasive imaging, these early percutaneous diagnostic procedures have largely been supplanted by Doppler sonography, computed tomography (CT), and magnetic resonance (MR) angiography. The focus of interventional radiology has since shifted to therapeutic interventions to reduce and mitigate the sequelae of portal hypertension. Classically, interventional radiology has been associated with the transjugular intrahepatic portosystemic shunt (TIPS) procedure, which was introduced in the 1980s with the advent of metallic stents and is now considered a mainstay in the management of portal hypertension (see Chapter 4). 1
This chapter focuses on special considerations regarding unique anatomical, technical, and physiological challenges in the percutaneous interventional treatment of portal hypertension.
5.2 Portal Vein Thrombosis
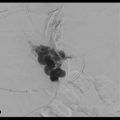
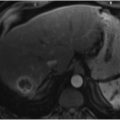
Portal vein thrombosis is a relatively rare condition that can occur in cirrhotic and noncirrhotic livers (▶ Fig. 5.1a,b). Etiologies of portal vein thrombosis include iatrogenic causes (prior portal vein catheterization), hypercoagulable states, trauma, tumors, postinflammatory conditions (pancreatitis), and postsurgical states.

Patients with cirrhosis and portal vein thrombosis are often asymptomatic, with the condition identified incidentally on imaging. Occlusion of the portal vein or its branches in noncirrhotic livers can cause localized alterations in hemodynamics; this occurrence accounts for less than 10% of all cases of portal hypertension. 2 These patients usually present with variceal bleeding, ascites, and abdominal pain. 3 Anticoagulation can be performed in cases of acute thrombosis, but this treatment is generally contraindicated in patients with active bleeding. Recanalization of the occluded portal vein by angioplasty and stenting via a transjugular or a percutaneous transhepatic approach can be performed to alleviate symptoms. Surgical shunting or devascularization is typically avoided in the cirrhotic patient to maintain transplant options in the future. 4
The technical approach to a thrombosed portal vein recanalization has been described by several authors. 4 , 5 , 6 , 7 , 8 , 9 , 10 These cases are undertaken using a variety of methods, including percutaneous transhepatic, transjugular, trans-splenic, or combined surgical transmesenteric approaches. Thrombosis of a portal vein can increase the technical complexity of creating a TIPS, especially in the setting of chronic occlusion and cavernous transformation. 4
When used with the results from previous cross-sectional imaging, intraparenchymal CO2 portography and direct puncture portal venography (▶ Fig. 5.2a) can be extremely useful in cases of portal vein thrombosis. 7 Newer techniques using CT and live fluoroscopic fusion for needle guidance may assist the operator with finding the appropriate orientation and anatomic position of the portal vessels for puncture (▶ Fig. 5.2b,c). The next step is to probe with a stiff guidewire and attempt to direct the wire toward the expected position of the main portal vein. Once the guidewire is advanced to the expected location of the portal vein, an angiographic catheter is inserted and a portogram may be performed (▶ Fig. 5.2d,e). If the occluded portal vein cannot be punctured, the ideal target may be a nonthrombosed intrahepatic portal vein branch or a collateral vein within the cavernoma (▶ Fig. 5.3a). If the portogram confirms correct positioning within the portal venous system, further intervention can be performed to recanalize the portal vein, including mechanical thrombectomy and pulse-spray thrombolysis, with or without stent placement (▶ Fig. 5.3b,c). The TIPS placement can then be completed in the usual fashion. In all cases, stent graft positioning should be intrahepatic, with the possibility for future transplant kept under consideration.


5.3 Budd–Chiari Syndrome and Veno-occlusive Disease
Budd–Chiari syndrome encompasses all obstructions to the hepatic vein outflow at the level of the hepatic vein and/or the inferior vena cava (IVC), including:
Hepatic venules.
Main hepatic veins.
Hepatic vein orifice–suprahepatic IVC.
Suprahepatic IVC–right atrium.
This causes symptoms of massive ascites, hepatosplenomegaly, abdominal pain, variceal bleeding, and jaundice from hepatic congestion, which, when left untreated, progresses to hepatic necrosis and fibrosis. 11
Hepatic venography and cavography should be performed to identify venous drainage. This is achieved by positioning a flush catheter below the infrahepatic IVC and performing a power injection of contrast at a rate of 20 mL/s for a total volume of 40 mL. Patients with Budd–Chiari syndrome may have complete occlusion of the hepatic veins with the classic “spider web” appearance on imaging (▶ Fig. 5.4) due to small draining collateral and recanalized veins.

The aim of treatment in these patients is to restore physiological flow to relieve hepatic congestion and prevent progression to irreversible liver damage. Initial therapy is often systemic anticoagulation. Endovascular options are also available and highly effective if the obstruction occurs over a short segment. 12 Acute thrombosis can be treated with catheter-directed thrombolysis or mechanical thrombectomy. 13 Functional stenosis in the vena cava or hepatic veins should be treated with angioplasty (and/or stents) if a significant gradient across the lesion exists (<4 mm Hg). 14 The use of intravascular ultrasound (IVUS) to plan and evaluate stent placement is helpful and is becoming more accessible to operators as utilization costs decrease.
Longer segments of venous occlusion are more difficult to reopen and are associated with high reocclusion rates. In these patients and those with disease progression despite anticoagulation, TIPS placement may be required to provide adequate outflow. 15 , 16 These cases present a challenge for interventional radiologists placing a TIPS, as there may not be a suitable vein for placement of the transjugular access sheath. Instead, the access system is preferentially wedged into the small hepatic vein stump for anchoring purposes (▶ Fig. 5.5a). As always, review of cross-sectional imaging is important to determine the anatomy and orientation of the portal vein in relation to the hepatic vein stump. Some radiologists prefer a left internal jugular vein access approach, as this approach may provide favorable working angles. 14 At this point, a small amount of contrast can be injected through the access system to confirm proper positioning in the hepatic parenchyma. A CO2 portogram should be performed to localize the portal vein (▶ Fig. 5.5b), and the needle should be torqued in the correct orientation to perform the transhepatic puncture (▶ Fig. 5.5c). The remainder of the TIPS creation follows the usual steps involving tract dilation and stent graft placement.

In the event that the portal vein cannot be localized with CO2, which is often the case as the obstruction tends to be postsinusoidal, access guidance with IVUS 15 or arterial portography can be used (▶ Fig. 5.6).

TIPS placement in this setting, as with other causes of portal hypertension, is preferable to surgical methods. Surgical portacaval shunts are often difficult to create in cases of Budd–Chiari syndrome because of overgrowth of the caudate lobe limiting access to the portal vein. 17 Additionally, the surgical shunt uses a high-flow retrohepatic cava because of extrinsic compression from the caudate lobe. Long-term survival after TIPS placement is excellent, with rates exceeding 90% at 5 years. 14
5.4 Direct Intrahepatic Portosystemic Shunt
Direct puncture of the main portal vein via the IVC (direct intrahepatic portosystemic shunt, or DIPS) is a method used in cases where the anatomy challenges the traditional TIPS placement approach. This can be seen in the setting of unfavorable hepatic or portal vein orientation, venous occlusion, extremely shrunken livers at risk for capsular perforation, and in the presence of hepatic parenchymal lesions. The presence of a tumor in the liver is considered a contraindication to the creation of a portacaval shunt using the traditional approach. This may stem from concerns regarding theoretical endovascular seeding of malignant cells during puncture attempts. 18
DIPS placement is technically challenging and requires careful planning with evaluation of preprocedural imaging. Review of axial multiphase CT or MR images is extremely useful in this regard. An optimal level for IVC puncture is determined below the level of the hepatic veins. Access is then obtained into the IVC via the jugular vein, and a needle with an exaggerated curve (nearly 90° angle) is used for the puncture (Rösch-Uchida transjugular liver access set, Cook, Bloomington, IN). The use of an IVUS probe from a femoral vein approach is advised to guide the puncture (▶ Fig. 5.7a). 19 The target is the main portal vein proximal to the bifurcation. Once access is obtained into the main portal vein, the rest of the procedure is completed in the typical fashion.

An alternative approach to DIPS creation via a percutaneous transhepatic approach is useful in patients with anatomy precluding them from conventional TIPS placement. With this technique, a 10-French sheath is initially placed in the IVC via jugular vein access. Transhepatic access into a branch of the right portal vein is obtained percutaneously using an 18- or 21-gauge Chiba needle (Cook) under US guidance. The needle is then advanced further into the IVC under imaging guidance (▶ Fig. 5.7b), either US or rotational fluoroscopy (cone-beam CT) with live fluoroscopy fusion. A 0.018- or 0.035-inch wire can then be passed through the transhepatic access and snared into the transjugular sheath. With access obtained between the IVC and portal system (▶ Fig. 5.7c), the shunt can then be created in the typical fashion (▶ Fig. 5.7d). Embolization of the transhepatic portal vein access tract with Gelfoam, coils, glue, and vascular plugs has been shown to decrease the risk of bleeding complications after the procedure (▶ Fig. 5.7e). 18
A transcaval approach may pose a higher risk of vascular injury and bleeding complications. The use of covered stents is critical in these patients, as extrahepatic puncture of the portal vein is common.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree