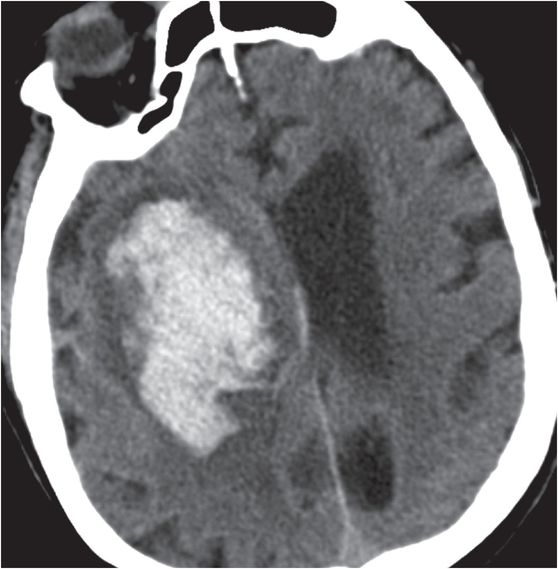
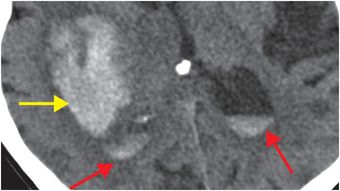
Diagnosis: Nontraumatic intraparenchymal hematoma (due to hypertension)
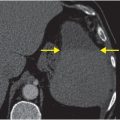
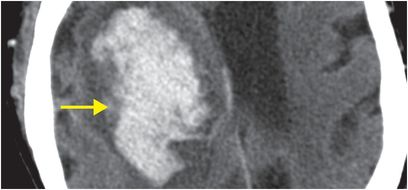
Axial unenhanced head CT shows a large right basal ganglia hematoma (yellow arrows), with layering blood in the lateral ventricles (red arrows). There is moderate mass effect from the hematoma.
Discussion
Overview of nontraumatic intraparenchymal hemorrhage (IPH)
Nontraumatic intraparenchymal hemorrhage (IPH) is the cause of 10% of first strokes and is a major cause of death and permanent disability. Clinically, IPH may manifest as a new neurologic deficit (mimicking ischemic stroke), seizure, headache, or altered consciousness.
Nontraumatic IPH is most commonly due to longstanding hypertension in elderly individuals. Chronic smooth muscle hyperplasia leads to replacement of smooth muscle with collagen, which predisposes to vascular ectasia and hemorrhage. The risk of hemorrhage, even without underlying hypertension, is increased in patients receiving anticoagulation therapy.
Less common causes of IPH include amyloid angiopathy, arteriovenous malformation (AVM), intracranial aneurysm, venous sinus thrombosis, cavernoma, hemorrhagic neoplasm, dural arterial–venous fistula (dAVF), hemorrhagic conversion of infarct, vasculitis, and cocaine use.
Imaging of nontraumatic intraparenchymal hemorrhage
Noncontrast CT is typically the first study performed in the emergent setting in a patient with a suspected intracranial hemorrhage.
Patterns of hemorrhage on initial noncontrast head CT, combined with clinical attributes such as age, presence of pre-existing hypertension, and treatment with anticoagulant medications, may suggest a specific etiology, but often, more advanced imaging (MR or CT angiography) is necessary to arrive at a specific diagnosis.
CTA is generally considered to be equivalent to invasive angiography for evaluation of vascular causes of intraparenchymal hemorrhage, such as AVM, dAVF, ruptured aneurysm, and venous thrombosis.
MR is better for evaluation of cavernoma, amyloid angiopathy, hypertensive encephalopathy, diffuse axonal injury, and hemorrhagic conversion of infarct all of which may present with multiple foci of susceptibility on gradient-recalled echo (GRE) MRI.
The MR imaging appearance of hemorrhage is dependent on the signal characteristics of blood degradation products, which progress in a predictable manner, allowing “dating”. This progression depends on intra-axial location and macrophage breakdown of blood products, which follows a predictable time-course. Extra-axial hemorrhage such as subdural hematoma cannot be dated in the same way.
Clinical synopsis
This 90-year-old male with a large basal ganglia hematoma due to hypertension was emergently intubated for hypoxic respiratory failure. He was unable to open his eyes, but was able to move his right thumb. Due to his poor prognosis, he was treated conservatively and died the following day.
Evolution of IPH
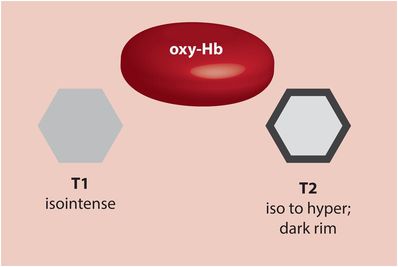
Hyperacute hematoma (oxyhemoglobin; 0–6 hours)
Up to a few hours after the ictus, the blood located centrally within a hyperacute hematoma contains intracellular oxyhemoglobin, which is isointense to brain on T1-weighted imaging and isointense to slightly hyperintense on T2-weighted imaging. T2-weighting characteristically features a hypointense peripheral rim, which is thought to be caused by peripheral deoxygenation.
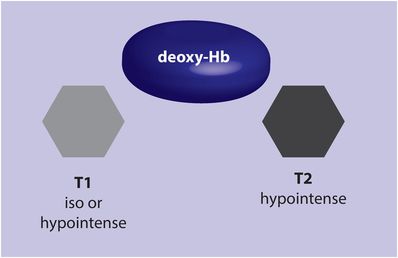
Acute hematoma (deoxyhemoglobin; 6–72 hours)
As the blood within the entire hematoma begins to deoxygenate, the appearance on T2-weighted imaging becomes uniformly hypointense due to predomination of deoxyhemoglobin. The appearance on T1-weighted imaging is iso- or hypointense to brain.
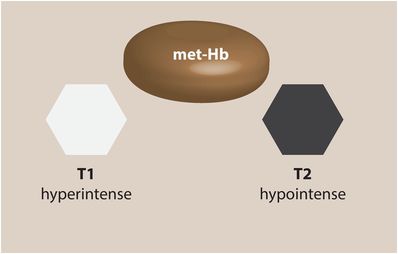
Early subacute hematoma (intracellular methemoglobin; 3 days – 1 week)
At this stage of hematoma evolution, the intracellular hemoglobin has been converted to methemoglobin, which causes dramatically shortened T1 times leading to a hyperintense appearance on T1-weighted imaging. The appearance on T2-weighted imaging remains hypointense, as intracellular methemoglobin has a strong paramagnetic effect.
Late subacute hematoma (1 week – several months)
After the red cells lyse, methemoglobin becomes primarily extracellular, and remains hyperintense on T1-weighted imaging. On T2-weighted images, however, the extracellular methemoglobin is also hyperintense, thought to be due to decreased protein concentration.
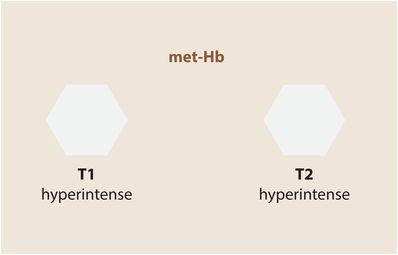
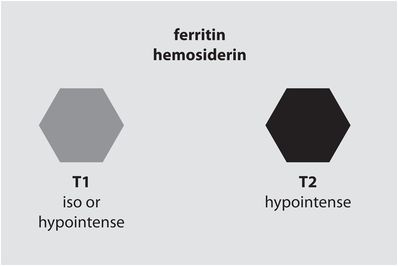
Chronic hematoma (several months and beyond)
Iron is permanently deposited as hemosiderin and ferritin, which remain trapped once the blood–brain barrier has been restored. The resultant strong susceptibility effects cause a very low signal on T2-weighted images. Chronic hematoma is isointense to slightly hypointense to brain on T1-weighted images. Chronic hematoma may also demonstrate peripheral enhancement.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree