Diagnosis: Vertebral artery dissection
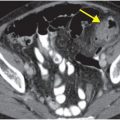
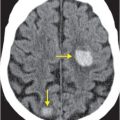
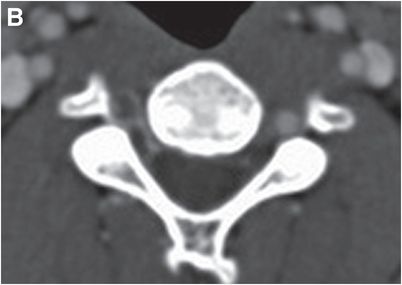
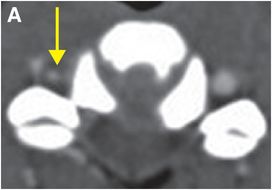
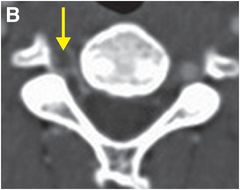
Axial CTA with IV contrast (A, B) shows decreased caliber of the right vertebral artery (arrow) in comparison to the left, with soft tissue density encasing the patent lumen.
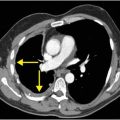
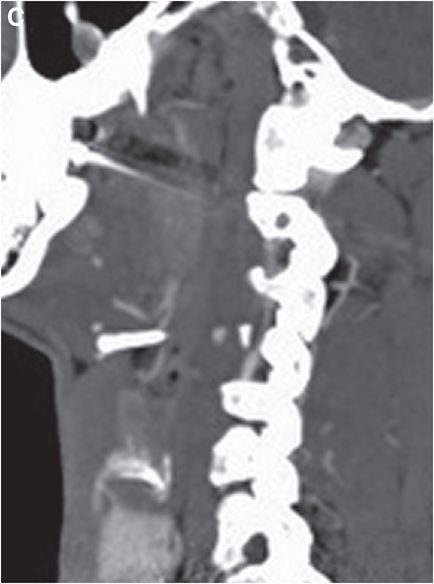
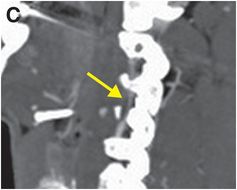
Sagittal CTA maximum intensity projection (MIP) of the right vertebral artery (C) shows eccentric stenosis of the mid right cervical vertebral artery (V2 segment), with soft tissue density along the stenotic segment (arrow).
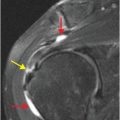
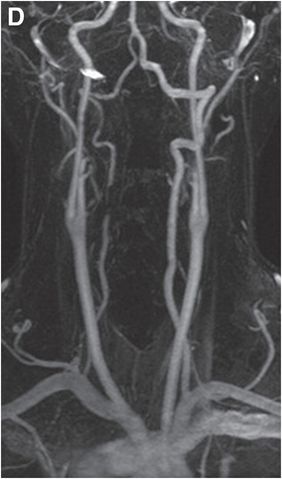
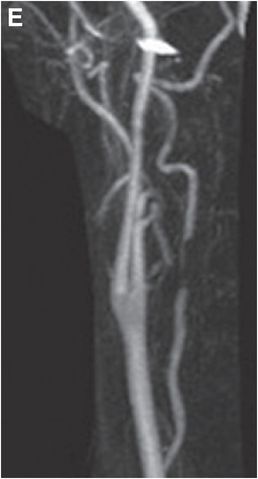
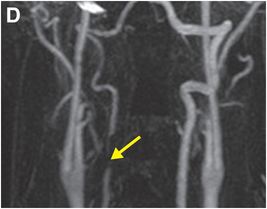
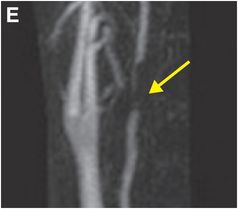
Coronal (D) and sagittal (E) gadolinium-enhanced MRA MIP images show near-complete loss of normal flow signal at the same level (arrow).
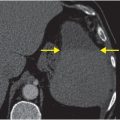
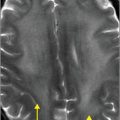
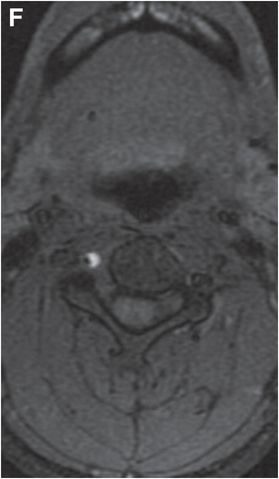
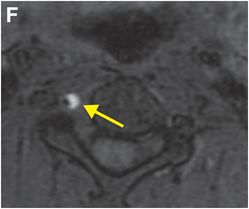
Axial T1 fat saturation MRI (F) shows intramural hematoma as a crescent-shaped focus of high signal (arrow) that narrows the patent lumen (dark flow void).
Discussion
Overview of vertebral artery dissection
In vertebral artery dissection, blood enters the media from the true lumen via an intimal tear. This creates a false lumen with an intimomedial flap that may cause vascular stenosis or occlusion. In subadventitial dissection, rupture of the vasa vasorum results in accumulation of blood within the subadventitial media, which may produce pseudoaneurysms or frank adventitial rupture. Both patterns may occur simultaneously.
Vertebral artery dissections may be spontaneous or result from major or minor trauma.
Prompt diagnosis is essential to commence timely treatment and minimize complications such as embolic infarcts.
Vertebral artery dissection is an important cause of stroke in younger patients. The incidence of spontaneous vertebral artery dissection is about 1 to 1.5 per 100,000 people per year, accounting for 0.4–2.5% of all ischemic strokes. In patients under 45 years of age, vertebral artery dissections cause up to 25% of strokes.
Clinically, patients may present with occipital headache and neck pain (70%) or with signs and symptoms related to ischemia of the vertebrobasilar system (60%), including nystagmus, ataxia, vertigo, or nausea. Less common symptoms include paresthesias or weakness from cervical spinal cord ischemia or cervical nerve root impairment.
The extracranial segments of the vertebral artery are thought to be more prone to dissection due to their increased mobility and proximity to bony structures. The horizontal V3 segment is particularly susceptible to traumatic dissection due to anchoring at the C2 foramen transversarium and the dura.
Imaging of vertebral artery dissection
Unenhanced CT is often the first imaging study ordered in cases of vertebral artery dissection, especially when the presenting signs and symptoms are nonspecific. CT may be normal or show evidence of infarction or, rarely, subarachnoid hemorrhage in cases of adventitial rupture related to intradural (V4 segment) vertebral artery dissection.
MRI/MRA and CTA are similar in sensitivity and specificity for vertebral artery dissection. Noninvasive imaging is generally preferred over conventional angiography except for rare situations in which endovascular intervention is being considered.
CTA findings may include luminal irregularity, vascular stenosis, occlusion, intimal flap, and the “suboccipital rind” sign. Non-enhancing soft tissue density adjacent to the lumen suggests intramural hematoma (IMH). The “suboccipital rind” sign, a segment of non-enhancing soft tissue density adjacent to the vessel lumen, may be the only abnormality of the V3 segment where dissections may not significantly narrow the lumen. Mimics of the suboccipital rind sign include normal veins of the vertebral artery venous plexus and suboccipital venous sinus that may parallel the course of the vertebral artery; however, the suboccipital rind sign may be distinguished from these normal venous networks by absence of enhancement after administration of contrast.
A normal anatomic variant that is occasionally mistaken for dissection is size discrepancy between the vertebral arteries. Congenitally small arteries may be distinguished from arteries with dissection by their uniform size from origin to terminus with a corresponding hypoplastic transverse foramen.
MRI findings in vertebral artery dissection include IMH, intimal flap, and luminal irregularity, with or without associated stenosis or occlusion. IMH appears as a crescent-shaped area of T1 shortening that surrounds or occludes the vertebral artery lumen. Axial T1-weighted imaging with fat suppression best depicts this finding by facilitating distinction of the fat that normally surrounds the blood vessel from the adjacent hematoma. While both fat and methemoglobin are inherently hyperintense on standard T1-weighted images, fat suppression accentuates the hyperintense hematoma, which is not saturated out. An important caveat is that acute or chronic intramural hematoma may be isointense to surrounding structures on T1 fat-suppressed imaging, as only subacute hemorrhage (containing methemoglobin) is hyperintense on T1-weighted images.
Areas of acute infarction due to any ischemic cause, including dissection, will show abnormal signal on diffusion-weighted MRI.
Angiographic imaging should be strongly considered in selected cases of cervical spine fracture, especially those that involve the transverse foramina. Connective tissue disorders such as fibromuscular dysplasia, Marfan syndrome, Ehlers–Danlos syndrome, and osteogenesis imperfecta type I all predispose to non-traumatic dissection.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree