6 Orbit and Globe
The paired orbits are pyramid-shaped cavities on either side of the ethmoid and sphenoid sinuses. The anterior cranial fossa lies above each orbit, the maxillary sinus below, the middle cranial fossa posterolaterally, and the temporal fossa anterolaterally.
Seven bones contribute to the bony orbit: the maxillary, frontal, lacrimal, and zygomatic bones, which are membranous in origin, and the sphenoid, palatine, and ethmoid bones, which are endochondral. The orbital plane of the frontal bone and the lesser wing of the sphenoid form the roof of the orbit. Portions of the frontal bone, the zygomatic bone, and the greater wing of the sphenoid bone form the lateral wall. The maxillary bone, zygoma, and orbital process of the palatine bone form the orbital floor. The medial wall is made up of the maxillary bone, lacrimal bone, ethmoid bone, lesser wing of the sphenoid bone, and frontal bone. The orbital apex is formed by the palatine bone and sphenoid bone.
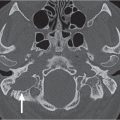
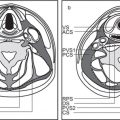
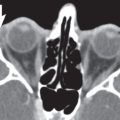
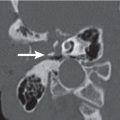
The bony orbit is bordered by the periosteum (periorbita), which is loosely adherent to the surrounding bones except at the trochlear fossa, lacrimal crest, and margins of the fissures and canals, where it is more tightly bound. Anteriorly, at the margins of the orbit, the periorbita is continuous with the orbital septum, a membranous sheet forming the fibrous layer of the eyelids. It defines preseptal and postseptal compartments. The preseptal space consists of the lids and anterior soft tissues of the orbit. The postseptal space (orbit proper) contains the globe, extraocular muscles, optic nerve sheath complex, lacrimal system, and various neural and vascular structures surrounded by well-organized adipose tissue with fibrovascular septa. It can be divided into four major anatomical components: the globe, the optic nerve sheath complex, the conal–intraconal area, and the extraconal area (Fig. 6.1).
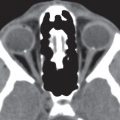
The globe, embedded in a fatty reticulum, has three coats and three fluid-filled intraocular chambers. It is roughly spherical in shape, measures about 2.5 cm long, is between 23 and 26 mm in equatorial diameter, and occupies approximately 20% of the total volume of the orbit.
The sclera, uvea, and retina compose the three layers of the globe. The sclera or outer layer is a fibrous capsule around the globe. It is contiguous with the transparent cornea anteriorly and the dural sleeve of the optic nerve posteriorly. The uvea or middle layer consists of the iris, ciliary body, and choroids. It is a vascular layer, which brings nutrients to the eye. It has an inner membrane (Bruch membrane) that separates the choroidal vessels from the retina. The retina consists of an outer pigmented layer and the sensorineural inner layer. The Tenon capsule envelops the eyeball from the margin of the cornea to the optic nerve and separates it from the central orbital fat. The episcleral space is a potential space between the Tenon capsule and the sclera that is filled with loose areolar tissue. Anteriorly, the conjunctiva covers the globe.
The lens apparatus divides the sphere of the globe into the anterior and posterior segments. The anterior segment, which is located between the cornea and lens and separated into the anterior and posterior chamber by the iris, is filled with an aqueous fluid, the aqueous humor. The posterior segment, located posterior to the lens and the ciliary muscle, is filled with vitreous humor. The thin hyaloid membrane envelops the vitreous body and is in contact with the posterior lens capsule, retina, and optic disk.
The optic nerve is not a true cranial nerve but rather an extension of the brain. The adult optic nerve is approximately 50 mm in length from the optic disk to the optic chiasm and 4 mm in diameter. The optic nerve can be divided into four different segments: the intraocular segment before the nerve penetrates the sclera, an intraorbital segment that traverses posteriorly in a slightly relaxed and undulating course through the orbital fat of the intraconal compartment, an intracanalicular segment within the optic canal, and an intracranial segment between the optic canal and the optic chiasm. The intraorbital portion is circumferentially invested by the pia–arachnoid, subarachnoid space, and dura mater, which blends with the sclera anteriorly and with the periosteum of the optic canal and the bony orbit posteriorly. The subarachnoid space that exists between the dura mater and the optic nerve pia is continuous with the intracranial subarachnoid space and reflects intracranial cerebrospinal fluid (CSF) pressure. The intracranial portion of the optic nerve is covered only by pia mater, as the dural sheath fuses with the periosteum of the optic canal.
The orbital cone consists of the extraocular muscles, which arise at the orbital apex from the annulus of Zinn and insert on the globe, and an envelope of fascia. This myofascial sling separates the retrobulbar space into the intraconal and extraconal compartments (Fig. 6.2). The extraconal compartment is the space outside the rectus muscle pyramid and contains the lacrimal gland and the lacrimal drainage apparatus, as well as cranial nerves (CNs) IV and V1 (n. lacrimalis, n. frontalis), extraconal branches of the ophthalmic artery, and portions of the superior and inferior ophthalmic vein, embedded in peripheral orbital fat.
The intraconal compartment is the space inside the rectus muscle pyramid, filled with central orbital fat, and contains, in addition to the optic nerve and nerve–sheath complex, the intraconal branches of the ophthalmic artery, superior ophthalmic vein, and CN III, V1, and VI.
Unlike the preseptal soft tissues, the retrobulbar space contains no lymphoid tissue or lymphatics.
The lacrimal system is composed of the lacrimal gland, the lacrimal drainage system (superior and inferior puncta, lacrimal canaliculi, common canaliculus, lacrimal sac, and nasolacrimal duct), and miscellaneous supporting structures. The orbital portion of the lacrimal gland lies in the bony lacrimal fossa, a post-septal extraconal space at the level of the zygomatic process of the frontal bone, just lateral to and superior of the globe adjacent to tendons of the levator palpebrae superioris and lateral rectus muscles. The smaller palpebral portion of the gland lies anterior to the orbital septum, where it projects onto the palpebral surface of the upper lid. These two portions of the lacrimal gland are connected by a small isthmus (its separation is not discernible on computed tomography [CT] scans). The nasolacrimal drainage apparatus is located within the bony lacrimal fossa in the preseptal portion of the inferomedial orbit at the suture of the frontal process of the maxilla and lacrimal bones, which, inferiorly, gives access to the nasolacrimal canal. It empties into the inferior nasal meatus.
The orbit communicates with multiple other compartments through various fissures and foramina. At the orbital apex, the optic canal forms a portal between the interior of the skull and the orbit and carries the optic nerve with its sheath, together with the ophthalmic artery and a complement of sympathetic nerves into the orbit. Sometimes the optic canal can project into the lumen of the sphenoid sinus or Onodi cells, and a bony dehiscence of its wall may be present.


The superior orbital fissure, formed from the greater and lesser wings of the sphenoid, connects the orbit with the middle cranial fossa, and the oculomotor (CN III), trochlear (CN IV), ophthalmic (CN V1), and abducens (CN VI) nerves, sympathetic fibers, an orbital branch of the middle meningeal artery, and the superior ophthalmic vein pass through this fissure.
The inferior orbital fissure communicates with the pterygopalatine fossa and infratemporal fossa. Veins passing through the fis-sure connect the orbital venous system with the pterygoid plexus. Parts of CN V2 (maxillary branch of the trigeminal nerve) and the infraorbital artery and vein also pass through this fissure.
The infraorbital groove and foramen transmit the infraorbital nerve and vasa infraorbitalis to the face.
The supraorbital foramen/incisure transmits the supraorbital artery and the superior ophthalmic vein (superior branch).
Near the suture between the frontal and ethmoid bones is the anterior and posterior ethmoid foramen. They transmit the anterior and posterior ethmoid nerves, arteries, and veins to the ethmoid sinus.
The nasolacrimal duct courses through the bony nasolacrimal canal and exits in the nasal cavity below the inferior turbinate.
CT demonstrates intrinsic contrast between bone, fat, and soft tissues. It is particularly useful in the evaluation of bony orbit, as well as pathology with potential calcification. The globe is well defined by CT. The higher density global wall is nicely contrasted by the very hypodense intraorbital fat posteriorly. The wall is uniform in width and density, except for a slight protuberance and change in density at the insertion of the optic nerve posteriorly (papilla). The various global layers cannot be differentiated on CT. The aqueous and vitreous chambers of the globe have a uniformly low density, whereas the lens stands out as a higher density structure in the anterior part of the globe. The CT density of the lens varies with the age, becoming progressively denser with age as it loses water content.
The extraocular muscles, lacrimal gland, Tenon capsule, intraorbital veins, ciliary muscle, optic sheath, and retinochoroidal layer significantly enhance with intravascular contrast media. The optic nerve normally does not enhance.
Proptosis is an abnormal protrusion of the globe beyond the orbital rim (> 21 mm anterior to the interzygomatic line on axial scans at the level of the lens). When associated with thyroid- associated orbitopathy, proptosis is sometimes called exophthalmos. Any space-occupying lesion within the orbit may cause proptosis. The proptosis of thyroid-associated orbitopathy and idiopathic orbital inflammatory disease is typically axial, or projected away from the center of the orbit. This differs from proptosis associated with localized masses elsewhere in the orbit, which may cause nonaxial proptosis. Nonaxial proptosis is a frequent finding with dermoid cyst, subperiosteal abscess, subperiosteal hematoma, vascular malformations (e.g., encapsulated venous vascular malformation [cavernous hemangioma], venous lymphatic malformation, and orbital varix), arteriovenous malformation (AVM; high flow lesion), carotid-cavernous sinus fistula, and aneurysm, vascular tumors (capillary hemangioma, hemangiopericytoma, hemangioendothelioma, and angiofibroma), meningioma, schwannoma, neurofibroma, rhabdomyosarcoma, leukemia, lymphoproliferative lesions of the orbit, Langerhans cell histiocytosis, malignant fibrous histiocytoma, fibrosarcoma, metastatic neuroblastoma, and meta-static carcinoma. Proptosis is an uncommon finding in children, but the vast majority of space-occupying lesions in the orbit causing proptosis in children are benign. These include congenital/developmental lesions (dermoid, epidermoid, teratoma, and neurofibromatosis type 1), inflammatory/infectious conditions (cellulitis, abscess, and idiopathic orbital inflammatory disease), benign mesenchymal tumors (osteogenic, chondrogenic, histiocytomatous, lipomatous, myxomatous, and rhabdomyomatous), vascular malformations and tumors of vascular origin (encapsulated venous vascular malformation [ cavernous hemangioma], venous lymphatic malformation, and capillary hemangioma), and neurogenic tumors (neurofibroma, plexiform neurofibromatosis, and glioma). The most common primary childhood orbital malignancy is rhabdomyosarcoma. Other orbital malignancies are malignant mesenchymal tumors, leukemia and lymphoproliferative lesions of the orbit, extension of retinoblastoma, secondary involvement by Ewing sarcoma, and metastases (neuroblastoma).
Enophthalmos refers to the relative depression of one globe back into the orbit. Any increase in size or volume of the orbit due to loss or defects of one or more walls of the orbit may cause enophthalmos. It is a finding with an orbita floor fracture and prolapse of fat and muscle into the maxillary sinus, or with a chronic sinusitis and the silent sinus syndrome. The absence of part of the sphenoid bone in neurofibromatosis type 1 may cause an enophthalmos. Breast carcinoma metastases may contract the orbital fat and cause enophthalmos. A phthisical, or end-stage contracted globe, may cause a pseudoenophthalmos. Cockayne syndrome may be associated with sunken eyes.
Leukokoria is an abnormal white, pink-white, or yellow-white pupillary light reflection that usually results from an intraocular abnormality and is seen most often in children. Common causes include retinoblastoma, medulloepithelioma, persistent hyper-plastic primary vitreous (PHPV), retinopathy of prematurity (ROP), congenital cataract, choroidal colobomas, uveitis, toxocaral sclerosing endophthalmitis, congenital retinal folds, Coats disease, retina dysplasia, retinal astrocytic hamartoma (associated with tuberous sclerosis and von Recklinghausen disease), organized vitreous hemorrhage, and long-standing retinal detachment. CT demonstration of a calcified mass in a globe of normal size may be helpful for differentiating retinoblastoma from alternative diagnosis (PHPV, ROP, Coats disease, and a variety of other nonspecific causes of leukokoria). In toxocaral endophthalmitis, the eye is of normal size and has no calcifications. In Coats disease, true calcifications are rarely seen. PHPV is associated with unilateral microphthalmia, and ROP with bilateral microphthalmia; calcifications are often absent.
Microphthalmia is defined as an eye measuring less than two thirds of its normal size, or < 16 mm in axial dimension in adults and children. It may be divided into simple and complex types, and further subdivided into primary (congenital) and secondary (acquired) forms.
An anatomically correct but small eye characterizes simple primary microphthalmos, unilaterally or bilaterally, with no concurrent anomalies. The orbital cavity is small. The extraocular muscles are thin.
Simple secondary microphthalmos may be due to intrauterine infection (toxoplasmosis, rubella, and cytomegalovirus [CMV]) or vascular disorders (PHPV and ROP). The globe tends to be small but normally shaped. In adults, simple secondary microphthalmos may also result from trauma or infection (tuberculosis [TB] and acquired immunodeficiency syndrome [AIDS]-related CMV infection). These cause scarring and inward retraction of the globe, eventuating in phthisis bulbi. The bony orbits and retroocular structures have normal size and configu-ration. A small globe is also found in optic nerve atrophy.
Complex microphthalmia is congenital with intraocular malformations and may be divided into those eyes with and without colobomas and cysts. Complex microphthalmos may be associated with concurrent malformations, such as basal encephaloceles, dysgenesis of the corpus callosum, and the median cleft syndromes, as well as a wide variety of congenital syndromes and chromosomal abnormalities.
Congenital microphthalmia is seen on CT scans as a small globe associated with a small, underdeveloped bony orbit. In acquired microphthalmia, usually unilateral and found after trauma, surgery, inflammation with disorganization of the eye, or radiation therapy, CT scans show a shrunken, often calcified globe, termed phthisis bulbi, in a normal developed orbit. Bilateral microphthalmia and cataracts are seen with congenital rubella, PHPV, ROP, retinal folds, Lowe syndrome, Norrie disease, and Warburg syndrome. Nanophthalmos is a condition in which both eyes are abnormally small but otherwise normal.
Macrophthalmia (enlargement of the globe) is most commonly the result of juvenile glaucoma or myopia. Its most severe form, buphthalmos, is caused by juvenile-onset glaucoma. Macrophthalmia has to be differentiated from proptosis, indicating the anterior protrusion of a normal-sized globe.
Ocular calcification is commonly found in normal and abnormal tissues. Idiopathic scleral calcification (senile scleral plaque near the insertions of lateral and medial rectus muscles) is seen in many patients older than 70 y of age. Ciliary body calcification may be seen after trauma, inflammation, or in teratoid medulloepithelioma of the ciliary body. Choroidal calcification often follows severe intraocular inflammation or trauma. Osteoma is an unusual but distinct cause of choroidal calcification peripapillary. Retinal astrocytic hamartoma (associated with tuberous sclerosis or neurofibromatosis), optic nerve head drusen (bilateral in 75%), and CMV retinitis may have retinal calcification. Tumor calcifications in the globe are characteristic of retinoblastoma. Meningioma of the optic nerve sheath infiltrating the posterior globe and hemangioblastoma (von Hippel–Lindau disease) may also produce intraglobal calcifications.
Extraglobal calcifications are most often of vascular origin (arteriosclerosis, phlebolith, encapsulated venous malformation, and AVM). Calcified hematoma, granuloma, and abscess also occur. The most common calcified tumor is a meningioma of the optic nerve sheath. Other calcified tumors are optic glioma, neurofibroma, neuroblastoma, lacrimal gland neoplasm, and dermoid. Lesions from the orbital wall and retinoblastoma with calcifications may occasionally extend into the extraglobal space.
Extraocular muscle enlargement (the maximum normal thickness in the belly of an extraocular muscle is 4.0 mm, measured in the axis perpendicular to the orbital wall) is a prominent feature of diseases such as thyroid-associated orbitopathy, myositis (idiopathic orbital pseudotumor, bacterial [from sinus extension], cysticercosis, trichinosis, and other, e.g., Lyme disease), vascular congestion (carotid-cavernous sinus fistula, dural AVM, superior ophthalmic vein or cavernous sinus thrombosis, and orbital apex mass), malignant neoplasm (metastases, adenoid cystic carcinoma, lymphoproliferative lesions of the orbit, and rhabdomyosarcoma), orbital lymphatic-venous malformation, trauma (edema and hemorrhage), acromegaly, and amyloidosis.
Optic nerve–sheath complex enlargement encompass a spectrum of disease entities, including primary and secondary neoplastic lesions (optic nerve glioma, optic nerve hemangioblastoma, optic nerve sheath meningioma, plexiform neurofibroma, leukemia, lymphoproliferative lesions, and metastases), inflammatory/infectious processes (idiopathic orbital pseudotumor, thyroid-associated orbitopathy, orbital sarcoidosis, TB, optic neuritis, and toxoplasmosis), trauma (perioptic hematoma), distended optic nerve sheath, and optic nerve sheath meningocele.
Enlargement of the superior ophthalmic vein can be associated with carotid-cavernous sinus fistula, dural AVM, superior ophthalmic vein or cavernous sinus thrombosis, orbital varix, orbital apex mass, thyroid-associated orbitopathy, idiopathic orbital pseudotumor, and normal variant.
Ocular detachment: there are basically three potential spaces in the eye that can accumulate fluid or blood, causing detachment of various layers of the globe: the posterior hyaloid space, the subretinal space, and the suprachoroidal space. Separation of the posterior hyaloid membrane from the sensory retina is referred to as posterior hyaloid detachment. It commonly occurs in the elderly with liquefaction of the vitreous, but it may also be present in children with PHPV.
Retinal detachment occurs when the sensory retina is separated from the retinal pigment epithelium. Retinal detachment may result from retraction associated with a mass, from a fibroproliferative disease in the vitreous, from endophthalmitis, or from retinal vascular leakage and hemorrhage.
Choroidal detachment is usually caused by ocular hypotony with accumulation of serous fluid or blood between the choroid and sclera, frequently after intraocular surgery or penetrating ocular trauma, and inflammatory choroidal disorders.
When considering orbital disease, it is helpful to localize the process to a compartment of the orbit, both for purposes of differential diagnosis and for therapeutic planning. The differential diagnosis of ocular lesions is discussed in Table 6.1 , optic nerve–sheath complex lesions in Table 6.2 , extraocular conal and intraconal lesions in Table 6.3 , and extraocular extraconal lesions in Table 6.4.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree