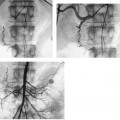
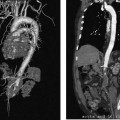
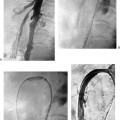
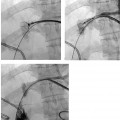
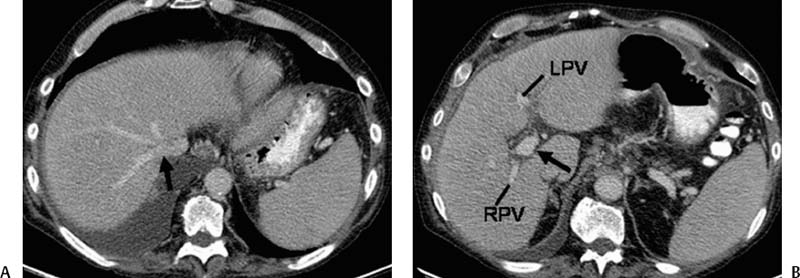
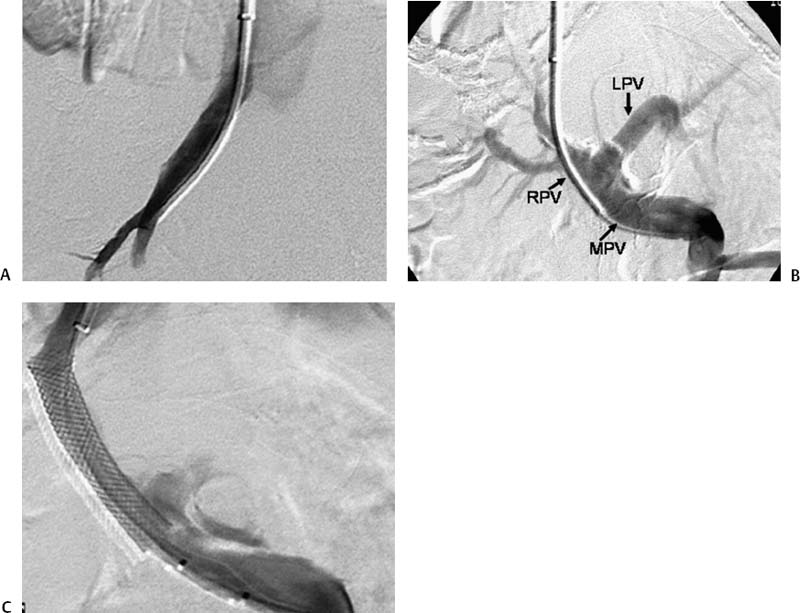
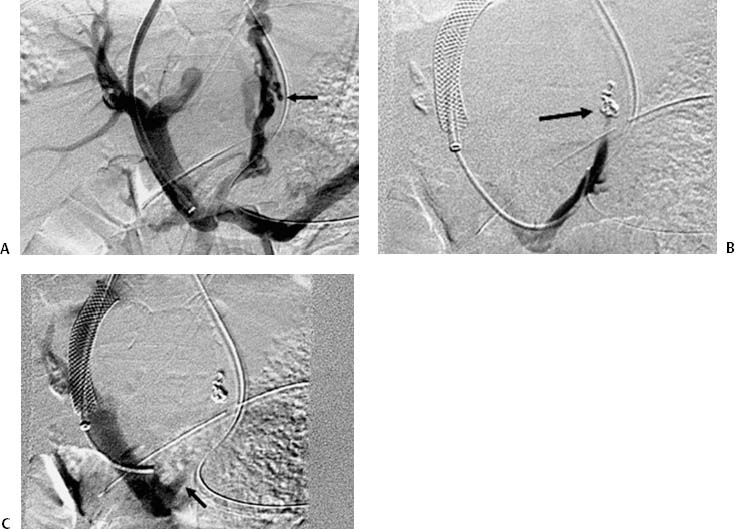
CASE 61 A 55-year-old male with hepatitis C virus cirrhosis presented with massive hematemesis requiring transfusion of 8 units of packed red blood cell transfusion over 8 hours despite endoscopic sclerotherapy by the gas-troenterology service. Bleeding esophageal varices were identified on endoscopic exam. Figure 61-1 A 55-year-old male with cirrhosis and variceal hemorrhage. Contrast-enhanced CT shows (A) patent hepatic veins at their confluence (arrow) with the inferior vena cava and (B) patent portal vein (arrow, main portal vein; RPV, right portal vein; LPV, left portal vein). A contrast-enhanced study showed patent portal and hepatic veins. Signs of portal hypertension were present, including splenomegaly, varices, and a right pleural effusion. Ultrasound examination (not shown) confirmed the portal vein patency. Variceal bleeding unresponsive to medical and endoscopic therapy. Two sets are widely used for TIPS creation, the Rosch-Uchida set (Cook, Bloomington, Indiana) and the Colapinto set (Cook, Bloomington, Indiana). A pediatric version of a TIPS set is available. The Rosch-Uchida set has a smaller puncture needle and thus can cause less damage in the blind punctures from the hepatic vein to the portal vein. The Colapinto set has a larger hollow-bore needle that is more effective in hard, fibrotic liver. These sets come with a long 11F vascular sheath in addition to the puncture needles. 0.035” conventional and stiff guidewires Angioplasty balloons Calibrated pigtail catheter Until recently, metallic stents used for TIPS creation were uncovered, self-expandable types such as the Wallstents (Boston Scientific, Natick, Massachusetts). In 2004, the Viatorr stent (W. L. Gore & Associates, Flagstaff, Arizona) was approved for TIPS creation. It is a self-expandable polytetrafluoroethylene-covered stent-graft with a distal 2 cm uncovered portion (Fig. 61-4). This stent is available in different lengths and diameters. Precise sizing is required with this stent. The uncovered portion is placed in the portal vein and the covered segment is placed in the tract and extends to the hepatic vein tributary with the inferior vena cava. At our institution, these stent grafts are used for TIPS creation. Access into the right internal jugular vein was performed using ultrasound guidance. The right hepatic vein was selected using an MPA catheter (Boston Scientific, Natick, Massachusetts), which has an angled tip, and an angled glidewire (Boston Scientific, Natick, Massachusetts). A hepatic venogram was performed to confirm position and assess patency of this vein. After confirmation, a Rosch-Uchida TIPS set (Cook, Bloomington, Indiana) was inserted into the hepatic vein over a stiff Amplatz guidewire. The 10-French (F) sheath from this set was positioned at the origin of the right hepatic vein. The curved sheath and needle/catheter system was used to direct anterior needle punctures into the right lobe of the liver. After each puncture, the needle was removed and the catheter was retracted while simultaneously gently aspirating with a small syringe. When blood return occurred, a small amount of contrast was injected to assess catheter tip position. Punctures were made until the right portal vein was entered. A guidewire was advanced into the portal vein, and directed toward the splenic vein. An 8-mm × 4-cm angioplasty balloon catheter (Ultrathin, Boston Scientific, Natick, Massachusetts) was used to dilate the hepatic parenchyma between the hepatic and portal veins. A pigtail marker catheter was placed into the portal vein and a portal venogram was performed to determine the length of the stent graft required to bridge the hepatic and portal veins. A Viatorr stent (W. L. Gore & Associates, Flagstaff, Arizona) was deployed over the wire to create the shunt. The stent was then dilated with the previously used angioplasty balloon. Cirrhosis is a common cause of portal hypertension in the United States and worldwide. It can lead to liver failure and portal hypertension and its sequelae of ascites and variceal hemorrhage, and it predisposes to development of hepatocellular carcinoma. Approximately 90% of patients with cirrhosis develop esophageal varices and bleeding will eventually occur in 30% of these patients.
Clinical Presentation
Radiologic Studies
Diagnosis
Treatment
Equipment
Transjugular Intrahepatic Portosystemic Shunt (TIPS)
Discussion
Background
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree