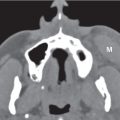
Congenital/developmental lesions |
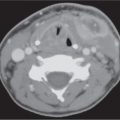
Choanal atresia
Fig. 7.2 |
Narrowed choana occluded by a bony plate (90%) or membranous web (10%), unilateral or bilateral. There is medial bowing of the posterior maxilla, bony thickening of the posterolateral nasal wall and posterior vomer, with both curving toward the obstructed choana. Hypoplasia of the nasal cavity is frequently associated.
Choanal stenosis with posterior nasal airway narrowed but not completely occluded is more common than true choanal atresia. Mucosal edema with choanal stenosis may imitate membranous atresia. |
Most common congenital abnormality of nasal cavity. The nasal cavity on one or both sides does not communicate with the nasopharynx. Half of these lesions are isolated, and half are associated with other abnormalities (e.g., cleft palate, Treacher–Collins syndrome, and cardiovascular and abdominal malformations). Congenital nasal piriform aperture stenosis (< 11 mm) is an uncommon cause of nasal obstruction in the newborn caused by bony overgrowth and medialization of the nasal processes of the maxilla. |
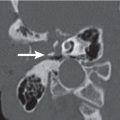
Nasopalatine cyst
Fig. 7.3 |
A sharply demarcated cystic structure is seen arising in the nasopalatine canal in the midline of the maxillary alveolar bone. When enlarging, it may extend posteri-orly into the hard palate (medial palatal cyst). Extensive nasopalatine cysts may also show remarkable nasolabial protrusion after penetration of the maxillary cortex. |
Most common nonodontogenic cyst in the maxilla. Most common symptom is swelling of the anterior part of the palate (mean age 51.9 y, predilect to male). |
Anterior cephalocele |
Heterogeneous, mixed density mass due to variable amounts of cerebrospinal fluid (CSF) and brain tissue extending through a base of skull defect, contiguous with intracranial brain parenchyma. The apertura is smooth and defined by a rim of cortical bone. In case of herniation through the foramen cecum, the foramen cecum is enlarged and the crista galli hypoplastic or absent. The nasal septum may appear deformed or truncated anteriorly. (The instillation of a low dose of intrathecal contrast before CT may aid in distinguishing a simple meningocele from an encephalocele; although MRI is the best modality for confirming the presence of brain tissue in a cephalocele.)
Frontoethmoidal cephaloceles are sincipital and subdivided into frontonasal, nasoethmoidal, and naso-orbital lesions. Transethmoidal, sphenoethmoidal, transsphenoidal, sphenoorbital, and sphenomaxillary cephaloceles are subtypes of basal cephaloceles. |
A cephalocele is the protrusion of intracranial contents, including meninges and brain matter, through a defect in the skull base. Cephaloceles may be congenital or acquired secondary to surgery, trauma, or due to spontaneous causes. Anterior cephaloceles account for 25% of all congenital cephaloceles. They may be visible from the outside (sincipital) or not visible (basal).
Rare congenital malformation. Affected patients typically present in the first year of life. Associated abnormalities are callosal hypogenesis, interhemispheric lipoma, dermoid, neuronal migration anomalies, colloid cyst, midline craniofacial dysraphisms, hypertelorism, microcephaly, microphthalmos, and hydrocephalus. |
Nasal dermoid/epidermoid |
Dermoid: Midline focal mass of fat density located anywhere from the glabella to the nasal columella. Findings that suggest intracranial extension include widened nasal septum, bifid septum, bifid perpendicular plate, bifid crista galli, interorbital widening, and defects in the cribriform plate. Dermal sinuses, complete or incomplete, are usually associated.
Epidermoid: Mass with attenuation close to water located in the subcutaneous fat of the nose. Epidermoids are more common at the tip of the nose or slightly lateral to the nose. |
Dermoid cysts are the most common midline congenital nasal masses. Nasal dermoids may be located anywhere from the glabella to the nasal tip and may extend intracranially. They may present with a nasal pit, dimple, or fistula containing a hair over the dorsum of the nose. Dermoids are slightly more common in male patients, whereas epidermoids have equal prevalence in both genders. They commonly become infected, may distort nasal growth, and are cosmetically unacceptable. |
Nasal glioma |
Well-circumscribed soft tissue mass, isodense to brain, located over nasal bones (extranasal glioma) or within nasal cavity (intranasal glioma). In patients with intranasal heterotopia, the foramen cecum may be deep and large, and the crista galli may be small or bifid. The anterior part of the nasal septum may be hypoplastic. With extranasal lesions, the nasal bones may be thinned. |
Rare, developmental mass of dysplastic neurogenic tissue sequestered and isolated from the subarachnoid space (“encephalocele” that has lost its intracranial connection). Usually identified at birth as a congenital subcutaneous blue or red mass along the nasal dorsum. Nasal obstruction may be present with intranasal glioma due to firm, polypoid submucosal nasal cavity mass. |
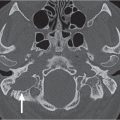
Protrusion of the internal carotid artery into the sphenoid sinus |
Internal carotid artery is one of the neighboring structures of the sphenoid sinus. It courses through the area surrounded by bony structures such as the sphenoid sinus wall or clinoid processes and can be identified as a bulge in the sphenoethmoidal cell wall. The grade of protrusion increases as the sinus grows larger. In some instances, the bony plate separating the sphenoid sinus from the artery may be very thin or absent. When the sphenoid sinus is highly pneumatized, the internal carotid artery can course freely through the sphenoid sinus cavity. |
One of the common anatomical variations of the sphenoid sinus, which can pose an endoscopic sinus surgical hazard if not recognized preoperatively. Protrusion of other neighboring structures (optic, maxillary, and vidian nerves) into the sphenoethmoid sinus lumen can also be seen. |
Congenital dacryocystocele (dacryocele)
Fig. 7.4 |
Well-circumscribed, rounded, hypodense, thin-walled lesion, anywhere from the lacrimal sac to the inferior aspect of the nasolacrimal duct at the inferior nasal meatus. There may be expansion of the nasolacrimal duct and erosion of adjacent bone. Unless infected, the density is homogeneous. If infected, there may be a rim of contrast enhancement around the cyst and enhancing inflammatory changes in surrounding soft tissues. |
A dacryocele (lacrimal sac mucocele) is a cystic expansion of the nasolacrimal sac or a diverticulum of the sac caused by a distal block of the lacrimal drainage system combined with a one-way valve effect at the proximal end of the system. It is almost always associated with intranasal cysts.
Dacryoceles are considered an uncommon congenital anomaly of the lacrimal drainage system and are usually present as a tense or fluctuant mass slightly nasal and inferior to the inner canthus in the first few days of life. Bilateral affectation is very rare. Most cases of congenital nasolacrimal duct cysts resolve spontaneously within 12 months. Secondary dacryocystitis, dacryopyocele, periorbital cellulites, and septicemia may ensue.
Adult nasolacrimal sac mucocele is an uncommon mass arising in the medial canthal region of the orbit of middle-aged patients with a history of prior trauma, surgery, and postinflammatory or neoplastic stenosis. The cystic expansion is associated with distal nasolacrimal duct obstruction and proximal obstruction at the junction of the common canaliculus and sac. Canaliculocele is an extremely rare cause of a medial canthal mass.
Nasolacrimal duct orifice cysts of the inferior nasal meatus are also rare acquired lesions. |
Maxillary sinus hypoplasia
Fig. 7.5 |
Unilateral small maxillary sinus with orbital enlargement. The bones between the maxillary sinus and the floor of the orbit and the roof of the orbit and the floor of the frontal fossa are depressed. The floor of the orbit remains rounded. The wall between the nasal fossa and the hypoplastic sinus is displaced laterally. Sinus may be aerated without thickening of mucoperiosteal membranes.
Maxillary sinus hypoplasia (MSH) shows three distinct patterns. Type I MSH characteristics are mild hypoplasia of the maxillary sinus, normal uncinate process, and a well-developed infundibular passage. Significant hypoplasia of the maxillary sinus, hypoplastic or absent uncinate process, and absent or pathologic infundibular passage are seen in type II MSH. Type III MSH is characterized by the absence of an uncinate process and cleftlike maxillary sinus hypoplasia. |
The maxillary sinus develops in the fourth month of intrauterine life as a mucosal evagination in the center of the middle nasal meatus. If development is arrested at this stage, aplasia or hypoplasia occurs with incomplete pneumatization into the malar eminence and maxillary alveolar ridge. MSH occurs more frequently in syndromes of craniosynostosis, osteodysplasia (Melnick–Needles), as well as in cases of Down syndrome (hypoplasia of the frontal sinus). Conditions such as severe infection, trauma, tumor, irradiation, and congenital first arch syndrome arrest the growth of the maxilla, resulting in a small (hypoplastic) antrum. |
Inflammatory/infectious conditions |
Acute rhinosinusitis |
CT findings that correlate with acute sinusitis include air–fluid levels with bubbly or strandlike secretions, nodular or smooth mucosal thickening with contrast enhancement, and complete sinus opacification by fluid or soft tissue (mucosal edema).
Imaging may reveal complications of sinusitis, such as secondary osteomyelitis, orbital complications, subcutaneous abscess (Pott puffy tumor), meningitis, epidural abscess, subdural empyema, and brain abscess. |
Acute inflammatory process of sinonasal mucosa lasting for less than 4 weeks. Most cases follow viral upper respiratory infections. The bacteria most often involved are Haemophilus influenzae and Streptococcus pneumoniae. Predisposing factors include upper respiratory infections, systemic disease, nasal masses, trauma, anatomical variants, dental infections, and allergic mucosal edema.
Manifests clinically as headache, local tenderness, facial pain, and purulent nasal drainage. |
Chronic rhinosinusitis
Fig. 7.6 |
In contrast with acute sinusitis, there is usually sclerosis and thickening of the affected sinus walls. Mucosal thickening may reflect inflammation or fibrosis. There is possibly dystrophic calcification of the mucosa. With coexistent acute disease, there may be enhancement of the inflamed mucosa. Signs of chronic rhinitis, including mucosal thickening, inflammatory polyps, and hyper-plasia of the turbinates, may also be evident.
Five different patterns of chronic rhinosinusitis may be described at CT:
• Infundibular pattern is mainly due to the presence of mucosal thickenings or isolated polyps along the ethmoid infundibulum with blockage of maxillary sinus drainage alone.
• Ostiomeatal unit pattern reflects obstruction of all drainage systems in the middle nasal meatus, leading to maxillary, frontal, and anterior ethmoid sinusitis. The most frequent causes are nonspecific mucosal thickenings and nasal polyps. This pattern may also be observed in the presence of benign or malignant neoplasms arising from the lateral nasal wall.
• Sphenoethmoidal recess pattern is rather rare, consisting of sphenoid sinusitis and/or posterior ethmoiditis secondary to sphenoethmoid recess obstruction.
• Pattern of nasal polyposis is characterized by bilateral involvement of the middle nasal meatus, as well as the ethmoid infundibular and paranasal cavities by inflammatory polyps.
• Sporadic pattern includes a wide list of different conditions, such as isolated sinusitis, retention cyst, mucocele, and postsurgical changes. |
Chronic sinusitis is an infection of the paranasal sinuses that persists beyond the acute stage (> 12 consecutive weeks’ duration) or fails to respond to therapy. May be bacterial, allergic, or fungal in nature. All but one pattern of chronic rhinosinusitis (sporadic pattern) are based on the obstruction of different mucus-drainage pathways. Often associated conditions are underlying anatomical variations and primary ciliary dysmotility.
All ages are affected. Common symptoms are facial pain and pressure, nasal obstruction, nasal discharge, hyposmia, and anosmia. |
Fungal sinusitis
Fig. 7.7 |
Localized Aspergillus sinusitis (mycetoma) usually a ffects a single sinus (maxillary > sphenoid > frontal > ethmoid sinuses). Presents with a focal polypoid mass within a partially or totally opacified sinus lumen with central areas of high density and/or fine, round to linear matrix calcifications. Thickened, inflamed mucosa at the periphery of the sinus may contrast enhance. The walls are thickened.
Chronic allergic hypersensitive aspergillus sinusitis presents with usually asymmetrical pansinusitis, hypodense contrast-enhancing mucoperiosteal thickening, polyps, and hyperdense sinus contents, sinus expansion, and facial deformity.
Acute invasive fungal sinusitis is most common in the maxillary and ethmoid sinuses, followed by the sphenoid sinus. The fulminant progressive disease presents with complete or partial soft tissue opacification of affected sinus, fungal colonization with increased density, absence of fluid levels, and multiple areas of focal bony destructions of sinus walls. Spread of sinus can extend into adjacent soft tissues and present with rhino-orbitocerebral disease. Vascular involvement can cause narrowing, dissection, and thrombosis. |
Aspergillus fumigatus and Mucoraceae are the most common offending organisms.
Acute invasive fungal sinusitis most often occurs in diabetic or immunocompromised patients with predisposing conditions (e.g., leukemia, bone marrow transplantation, severe malnutrition, or end-stage renal disease). Serious disease.
Noninvasive continuous forms may occur in an otherwise healthy patient after change in local sinus microenvironment (sinus surgery, postradiation, marijuana smoking). Asymptomatic or mild sensation of a chronic rhinosinusitis. |
Infectious granulomatous sinonasal disease |
Nodular or tumorlike masses in the nasal cavity and/or paranasal sinuses, commonly associated with bony destruction. External nasal involvement may also be present. |
Bacterial infections with granulomatous lesions of the sinonasal cavities include actinomycosis, nocardiosis, tuberculosis, syphilis, leprosy, and glanders. Rhinoscleroma is a tumorlike expansion of the nose and upper lip, seen more commonly in Africa, Central and South America, and Eastern Europe. It is caused by Klebsiella rhinoscleromatis. It can also involve the nasopharynx, larynx, trachea, bronchi, middle ear, and orbit. |
Cocaine nose |
Granuloma of the nasal septum, which eventually may be eroded. Nonspecific mucosal inflammation of the nasal cavity and/or paranasal sinuses may be a ssociated. The nasal septal destruction, loss of the structural integrity of the nasal cavity, and hard palate defect are recognized as a local complication. |
Secondary to necrotizing vasculitis with prolonged cocaine abuse. |
Wegener granulomatosis
Fig. 7.8 |
Favors nasal cavity (septum, turbinates) over sinuses. Presents with usually bilateral, irregular mucosal thickening and soft tissue nodules with contrast enhancement. Extensive bony destruction may occur without associated large soft tissue mass. Nasal septum perforation is common. May extend through hard palate. Orbital extension is the most common extrasinonasal site.
As the disease becomes chronic, the walls of the residual paranasal sinuses (particularly the maxillary sinus) become markedly thickened, while the sinus volume is gradually reduced, and the nasal septum may completely disappear. |
Systemic necrotizing granulomatous vasculitis that may also affect lungs and kidneys in addition to the sinonasal involvement. The antineutrophil cytoplasmic autoantibody assay is usually positive.
Nasal manifestations of allergic granulomatosis and angiitis (Churg–Strauss syndrome) are similar to Wegener granulomatosis.
Sarcoidosis may also present as granulomatous sinonasal disease with external nasal involvement and mucosal thickening of the septum and turbinates. |
Retention cyst |
Smooth, spherical mass of homogeneous water density adherent to the sinus wall. The mass does not enhance. Most arise in the floor of the maxillary antrum. A persistent rim of air and lack of sinus expansion distinguish the retention cyst from a mucocele. Retention cysts are usually not associated with mucosal thickening and edema. |
Retention cyst, the most common mass of the maxillary sinus, results from the obstruction of ducts draining mucous glands; considered a complication of sinusitis.
Retention cyst is most often asymptomatic. If large enough, it may become symptomatic by obstructing the sinus drainage. |
Sinonasal polyposis |
Multiple polypoid mucoid or soft tissue masses within the nasal fossa and paranasal sinuses. Polyps may be hyperdense with increased protein and decreased water content or colonization with fungal agent. Increased linear densities are due to trapped desiccated mucus. Curvilinear mucosal enhancement surrounding polyps is seen after intravenous (IV) contrast. Deossification of bony septa in ethmoid and nose, nasal cavity and sinus wall remodeling and expansion, enlargement of the infundibulum, lamina papyracea bulging into orbits, and even intracranial extensions are noted in severe cases (may have mucoceles associated). |
The most common sinonasal masses develop from chronic nonneoplastic inflammatory swelling of the mucosa. Predisposing factors include chronic rhinosinusitis, allergy, cystic fibrosis, Kartagener syndrome, asthma, and aspirin sensitivity. Polyposis and allergic fungal sinusitis are frequently seen in association.
Most commonly seen in adults; 60% are male. Patients present with nasal stuffiness and sometimes hypertelorism. |
Antrochoanal polyp
Fig. 7.9a, b |
A well-defined, homogeneous, low-density, mucuslike mass may be seen occupying the maxillary sinus, ipsi-lateral nasal cavity, and nasopharynx. Central increased density may be related to chronicity of polyp or fungal colonization. The maxillary ostium and infundibulum are considerably enlarged by passage of the dumbbell-shaped, nonenhancing polypoid lesion into the nose. |
Inflammatory, solitary, peculiar variant of sinonasal polyp without significant allergic pathophysiology. Arises from the maxillary antrum; represents 3% to 6% of all sinonasal polyps.
Most common in teenagers and young adults; predilection to male. Patients present with unilateral nasal obstruction, nasal drainage, cheek pain, and headaches. Bilateral obstructive maxillary sinusitis may be associated.
Nasochoanal, sphenochoanal, and ethmochoanal polyps are less common variants. |
Mucocele
Fig. 7.10 |
Airless, opacified, mucus-containing, nonenhancing enlarged and distorted sinus with smooth-walled expansion. High-density areas within the low-density or soft tissue density of the mucocele may be related to inspissated secretions or fungal colonization. Peripheral enhancement may suggest superinfection (mucopyocele). The sinus walls are remodeled and may even be thinned (thin peripheral rim of expanded bone) or focally absent due to pressure atrophy and erosion, but frank bone destruction and lytic changes are uncommon. Persistent expansion of the sinus is frequently associated with extension into the orbit or skull base and mass effect. In chronically infected mucoceles, a surrounding zone of sclerosis may be evident. |
Most common expansile lesion of paranasal sinuses. Frontal sinuses are most frequently involved (65%), followed by the ethmoid sinus (25%), maxillary sinus (5%–10%), and sphenoid sinus (2%–5%). Results from chronic ostial obstruction of affected sinus from inflammation, trauma, surgery, or any space- occupying sinonasal mass lesion; may occur in septated sinuses and anatomical variants (concha bullosa, pneumatized crista galli, uncinate process, etc.).
Most common in adults. Presenting symptoms depend on site of involvement, regional compression, and complications. |
Silent sinus syndrome
Fig. 7.11 |
Opacified, fully developed maxillary sinus with retraction and inward bowing of the orbital floor and the other sinus walls into sinus lumen. Uncinate process is apposed to inferomedial orbital wall, occluding maxillary sinus infundibulum. The adjacent middle nasal meatus is enlarged with varying degrees of lateral retraction of the middle turbinate. The decrease in sinus volume is associated with increased orbital volume and hypoglobus. The retroantral fat is widened. |
Most cases are idiopathic, without a prior history of trauma or surgery. Characterized by painless enophthalmos associated with chronic maxillary sinus atelectasis after infundibular occlusion, seen in adults between the fourth and fifth decades. Symptoms related to sinusitis may or may not be present. |
Cholesterol granuloma |
CT features of the paranasal sinus cholesterol granuloma are of two types: a variety in which the changes are entirely nonspecific and cannot be differentiated from inflammatory or allergic sinus disease, and a more characteristic appearance of a cystlike lesion within the sinus cavity accompanied by expansion of the bony walls involved.
CT of orbitofrontal cholesterol granuloma shows a lobular soft tissue expansile mass, isodense to brain, without contrast enhancement. Orbitofrontal cholesterol granulomas have a tendency to concentrate their expansion about the lateral margins of the frontal sinus affecting the superolateral orbital wall and adjacent soft tissues with a clear-cut area of osteolysis in the lateral part of the supraorbital ridge and frontal bone, characteristically extending into the zygomatic process as far as the frontomalar suture. Absent or minimal surrounding sclerosis. |
Cholesterol granuloma is the result of a hemorrhagic foreign body response elicited by cholesterol crystals; usually occurs in the temporal bone (middle ear cavity, mastoid, and petrous apex). Cholesterol granuloma arising from the paranasal sinus is uncommon and occurs in the maxillary antrum, sphenoidal sinus, and frontal bone.
It usually occurs in young or middle-aged men.
Cholesterol granuloma of the maxillary sinus is usually an incidental finding. An expansive lesion of the sphenoid sinus may present with progressive visual deficit. The most common symptom of orbitofrontal lesions is gradual proptosis. |
Benign neoplasms |
Osteoma |
Sharply delineated round or lobulated mass of bone attenuation arising from and confined to bone or protruding into a sinus. Ivory osteomas are denser than adjacent bone. Non-ivory-type (fibrous) osteomas contain both dense calcifications and mildly calcific soft tissue. Larger frontal osteomas may invade the posterior wall of the sinus, leading to CSF leak, pneumocephalus, or subdural empyema and brain abscess. Osteomas also may occlude the sinus ostia, causing sinusitis or mucocele formation. |
In the craniofacial skeleton, osteomas may be found in all sites but are most common in the frontal and ethmoid sinuses. Occur as a single lesion but may be associated with Gardner syndrome and multiple craniofacial osteomas.
Osteomas are more common in men, reported in all ages (rare under age 10). Usually asymptomatic. Symptoms associated with sinus osteomas include headaches, facial swelling or deformity, and ocular disturbances. |
Osteoblastoma |
Osteoblastoma appears as a well-defined, round, expansile lesion with a prominent calcified rim. The central portion may appear similar to ossifying fibroma as a solitary cystlike or solid soft tissue lesion, with or without mineralized components. Osteoblastomas may show moderate to marked enhancement. |
Uncommon osseous neoplasm. Head and neck sites of involvement include the mandible (most common site), maxilla, temporal bone, orbit, and paranasal sinuses. Osteoblastomas occur more often in men; most patients are younger than 30 y. Clinical symptoms include pain, facial swelling and asymmetry, and loosening of teeth. |
Chondroma |
Multilobulated, sharply demarcated soft tissue mass; can be hyperdense or hypodense relative to brain. Contrast enhancement is usually minimal. Calcifications within the tumor matrix are not often seen. These lesions tend to be expansile and can remodel bone.
A distinction between a benign chondroma and a low-grade chondrosarcoma is not possible on sectional imaging. |
Chondromas of the sinonasal tract are rare. Most frequent sites of occurrence include the nasal cavity (septum), the ethmoid sinus, and the nasopharynx. Most patients are younger than 50 y, and there is equal gender predilection. Patients may present with nasal obstruction, an enlarging, painless mass, proptosis, and headaches. |
Ossifying fibroma
Fig. 7.12 |
Unilocular, well-defined, expansile mixed soft tissue and bone density lesion. The low-attenuation fibrous center is surrounded either by a thick bony wall or a thin “eggshell” periphery of bone. Fibrous areas may show subtle contrast enhancement. |
Monostotic lesion of osteogenic origin, occurring primarily in the mandibular premolar/molar region of women in the third and fourth decade, can occur in the craniofacial skeleton and involve the adjacent sinuses (maxillary, ethmoid, and frontal sinuses). May obstruct sinus drainage pathways, result in cosmetic deformity, and extend intracranially. |
Schwannoma |
Schwannomas appear as well-defined ovoid to fusiform, homogeneous soft tissue mass, hypodense relative to skeletal muscle, and reveal moderate to marked contrast enhancement. The larger lesions, however, may be inhomogeneous as well as cystic. |
Neurogenic tumors are infrequent in the sinonasal cavities (nasal fossa, maxillary and ethmoid sinuses). They may arise from the first and second division of the trigeminal nerve and from the autonomic nerves (the olfactory nerve has no Schwann cells and therefore cannot be the site of these tumors). |
Hemangioma |
Well-defined, lobular, diffusely enhancing soft tissue density mass. May cause bone remodeling and septal deviation. |
Nasal hemangiomas arise from the nasal septum or vestibule and are of the capillary type. Only a few arise from the lateral wall of the nose, and these are usually cavernous. In the paranasal sinuses, hemangiomas are even rarer. Patients (with a wide age range from infancy to late adulthood) present with epistaxis and nasal obstruction. |
|
The CT appearance of intraosseous hemangiomas is variable and most commonly shows a characteristic sharply marginated expansile, rounded, well-defined bony lesion with intact, thinned, smooth inner and outer cortices and a sunburst or spoke-wheel-type pattern of radiating trabeculae. “Soap bubble” and “honeycomb” configuration may also occur. There is no associated periosteal reaction or soft tissue mass. |
Intraosseous hemangiomas of the facial bones are rare and most commonly arise in the maxilla, zygoma, mandible, and nasal bone. Patients usually present in the fourth decade of life, with a female predominance. Symptom: firm, nonpainful swelling. |
Juvenile angiofibroma
Fig. 7.13 |
Intensely enhancing lobular, well-circumscribed, soft tissue mass centered at the sphenopalatine foramen with multiple surrounding extensions into the nasal cavity, nasopharynx, paranasal sinuses, pterygopalatine fossa, masticator space, orbit (through the inferior orbital fissure), and intracranially (20%–36%; via superior orbital fissure, vidian canal, and foramen rotundum). Juvenile angiofibroma may cause adjacent compressive deossification or remodeling with enlarged ipsilateral nasal cavity and widening of the pterygopalatine fossa with anterior bowing of the posterior maxillary sinus wall (antral sign). |
Rare, histologically benign, highly vascular, nonencapsulated, locally aggressive tumor arising from the fibrovascular stroma on the posterolateral nasal wall adjacent to the sphenopalatine foramen.
Occurs almost exclusively in male adolescents with unilateral nasal obstruction and epistaxis, anosmia, serous otitis media, and pain. |
Odontogenic neoplasms |
|
Odontogenic tumors are neoplasms of the maxilla and mandible that originate from tooth-forming epithelial, mesenchymal tissue, or both. Clinically, they are generally asymptomatic but can cause bony swelling, tooth movement, tenderness, and pain when the bone is resolved. |
|
Ameloblastoma: Enhancing, expansile, uni- or multi-cystic, low-density mass with “bubbly” pattern, internal osseous septa, usually no calcifications in matrix, scalloped borders, and thinned cortical margins with focal areas of dehiscence. Extraosseous extension is rare. Unerupted molar tooth and resorption of adjacent tooth roots association is common. No evidence for perineural spread. Maxillary ameloblastomas occur most often in the molar-premolar region and can involve the adjacent maxillary sinus. |
Most common odontogenic tumor (35%), mandible to maxilla ratio = 5:1. Twenty percent of ameloblastomas are thought to arise from dentigerous cysts. Most commonly presents at age 30 to 50 y with painless, slow-growing mandibular mass, loose teeth, bleeding, and trismus. Maxillary lesions may present with nasal obstruction. Malignant transformation is rare (1%). |
|
Odontogenic fibroma: Well-defined, expansile, unilocular, radiolucent lesion, mainly in the mandibular molar area or in the maxilla, associated with sharp resorption of the roots of adjacent teeth. Calcification is rare. |
Rare disease, found in individuals age 20 y and younger. |
|
Odontogenic myxoma: Enhancing, expansile, multilocular lesion with straight and curved bony septa (“tennis racket” appearance), often accompanied by bulging of the bony cortex, which may be barely preserved, and inclination of adjacent teeth. |
A locally aggressive tumor, occurring only in the jaws. This tumor develops in the third decade of life, with a slightly higher prevalence among women. |
|
Odontomas: Well-demarcated mass with amorphous areas of calcification (complex odontoma) and/or malformed teeth (compound odontoma) a rranged in a disorderly pattern. A narrow radiolucent zone, along with a sharply defined bony rim representing the wall of the odontoma, frequently surrounds the tumor. |
Hamartomatous malformation of odontogenic tissue. Compound odontoma is the more frequent variety and occurs most commonly in the maxillary incisor or canine region. Complex odontoma resembles a mature cementoma but is seen more often in the mandibular molar regions, develops in childhood and adolescence, and is more radiodense. |
Schneiderian papilloma |
Inverted papillomas usually originate from the lateral nasal wall in the vicinity of the middle turbinate. They show a unilateral bulky, polypoid soft tissue mass with variable enhancement pattern, from diffuse to heterogeneous, that involves the nasal vault and extends centrifugally into the adjacent sinuses, nasopharynx, and orbit. Rarely, they violate the meninges and intracranial structures. Foci of coarse calcification are occasionally present. They cause local bone remodeling and a unilateral ostiomeatal unit obstructive pattern of sinus opacification (obstructed sinus secretions do not enhance). Bilateral up to 13% due to transseptal extension. |
Schneiderian papillomas are uncommon, representing 0.4% to 4.7% of all sinonasal tumors. Inverted papillomas account for 70% of sinonasal papillomas. This epithelial tumor of nasal mucosa is named for its characteristic endophytic rather than exophytic growth. Although histologically benign, they are locally aggressive tumors. An underlying or coexisting squamous cell carcinoma is present in 5.5% to 27% of cases. Peak incidence: 40 to 70 y, with male predominance. Patients present with nasal obstruction and discharge, epistaxis, anosmia, headache, and pain. Cylindric cell papillomas originate primarily from the lateral nasal wall.
Fungiform papillomas arise on the nasal septum in young males and have a verrucous appearance. |
Benign mixed tumor (pleomorphic adenoma) |
Benign mixed tumors usually arise from the nasal septum, the lateral nasal cavity wall or turbinate, with secondary extension into the maxillary sinus. They appear as a broad-based or pedunculated, polypoid, well-demarcated, expanding intranasal soft tissue mass, generally associated with bone destruction. |
Benign mixed tumors (pleomorphic adenomas) are the most common benign salivary-type neoplasms of the sinonasal tract.
Patients (median age 20–60 y, equal gender distribution) usually complain of nasal obstruction, mass sensation, or epistaxis. |
Malignant neoplasms |
Squamous cell carcinoma
Fig. 7.14 |
Large, poorly marginated, heterogeneous soft tissue density mass, with minimal to moderate contrast enhancement and extensive irregular destruction of adjacent bone. The tumor may show bone fragments or areas of punctate calcifications. Associated sinusitis or retained secretions are common due to ostial obstruction. Invasion of the retroantral fat pad and pterygopalatine fossa, maxillary alveolar ridge, buccal space, hard palate, and subcutaneous tissues of the cheek and nose occurs frequently. These tumors also have a propensity to grow insidiously toward the anterior and central skull base and orbits. Regional lymph nodes (retropharyngeal, submandibular, and/or jugulodigastric) are involved in 15% at presentation. |
Malignant tumors of the nasal cavity and paranasal sinuses are relatively rare (3% of all head and neck tumors). Squamous cell carcinoma accounts for 80% of sinonasal malignancies. Twenty-five to 60% of these carcinomas involve the maxillary antrum; however, the maxillary sinus is secondarily involved by direct extension in 80% of patients. The nasal cavity is the site of origin in ~30% of cases, the ethmoid air cells in 10%, and the sphenoid and frontal sinus in < 2%.
These are typically seen in patients who range in age from 60 to 70 y, more commonly in men. They are often clinically silent until advanced. First symptoms are usually from secondary obstructive sinusitis.
Staging:
T1: Tumor limited to antral mucosa.
T2: Confined to suprastructure mucosa without bone destruction, or infrastructure with inferior or medial bony wall destruction.
T3: Invades skin of cheek, posterior wall maxillary sinus, floor of medial wall orbit, masticator space, pterygoid plates, or ethmoid sinus.
T4: Invades orbit or cribriform plate, posterior ethmoid or sphenoid sinuses, nasopharynx, soft palate, or skull base. Staging criteria are based on a theoretical plane joining the medial canthus of the eye with the angle of the mandible (Ohngren line). The area anteroinferior to this line is referred to as the infrastructure; the superoposterior is called the suprastructure of the maxillary antrum. |
Glandular tumors of the sinonasal cavities
Fig. 7.15 |
Well-circumscribed to poorly defined soft tissue density mass. Attenuation within the tumor is usually inhomogeneous due to cystic degeneration, necrosis, or serous and mucous collections; with diffuse, often heterogeneous contrast enhancement. Bone changes include bone remodeling, sclerotic reactions, erosion, and destruction. These tumors have a propensity to grow insidiously toward the anterior skull base and orbit. Perineural tumor spread may appear as obliteration and enlargement of the nerve sheath.
Distant metastases are more common than lymph node involvement. |
Tumors of minor salivary gland origin and other glandular neoplasms constitute 4% to 10% of malignant neoplasms of the sinonasal cavities. They can occur anywhere within the sinonasal cavities. However, they frequently arise from the minor salivary glands within the palate and then extend into the nasal cavity or paranasal sinuses. Adenoid cystic carcinoma is the most common malignant tumor of the minor salivary glands (47% maxillary, 32% nasal cavity, 7% ethmoid, 3% sphenoid, and 2% frontal). The others are adeno-carcinoma, mucoepidermoid carcinoma, acinic cell carcinoma, and carcinoma ex pleomorphic adenoma. Adenocarcinomas more frequently arise in the ethmoid and are common in patients with occupational exposure to wood and leather dust.
Age distribution shows a peak occurrence in the sixth and seventh decades, with a marked predominance of men. Low-grade tumors tend to present in younger patients. Patient often presents when the tumor is at an advanced stage with symptoms of obstruction, rhinorrhea, and epistaxis. Associated dull pain, paresthesia, or nerve paralysis due to perineural spread is highly suggestive of adenoid cystic carcinoma. |
Merkel cell carcinoma |
Hypervascular, homogeneously and massively enhancing mass expanding the nasal fossa with aggressive destruction of the nasal septum, intersinonasal wall, and orbital wall. No calcifications. |
Highly aggressive neoplasm of neuroendocrine origin with poor prognosis. This rare tumor affects older patients (mean age 70 y), with a slight female predominance. The majority of the tumors are found on sun-exposed sites. Involvement of the nasal cavity and palatal mucosa is very uncommon. |
Olfactory neuroblastoma (esthesioneuroblastoma) |
Typically presents as a unilateral nasal homogeneous soft tissue mass with moderate to marked enhancement, often near the superolateral nasal wall near the frontoethmoidal complex. When large, the tumor may be inhomogeneous with areas of small or large cystic components. Punctate calcifications within the tumor matrix are unusual. Olfactory neuroblastomas have a marked propensity for crossing the cribriform plate and extending dumbbell-shaped intracranially. Other directions of tumor extension associated with bone remodeling mixed with destructive bone changes include ipsilateral ethmoid and maxillary sinuses. Orbital involvement is late. |
Rare malignant nasal neoplasm of neuroectodermal origin that arises from the olfactory epithelium in the cribriform region, the upper third of the nasal septum, and along the superior and supreme nasal turbinates. They have a bimodal age distribution, presenting in boys and middle-aged adults (slight male predominance). Patients present with long-standing unilateral nasal obstruction and repeated episodes of epistaxis, anosmia, rhinorrhea, headache, pain, and ocular disturbances; 20% have malignant cervical nodes at presentation. |
Osteosarcoma
Fig. 7.16 |
Poorly defined lesion with aggressive bone destruction, heterogeneous with ossified and nonossified soft tissue components, aggressive periosteal reaction with perpendicular spiculated pattern, and associated soft tissue extension; with enhancement of solid components after contrast. |
Only about 7% of osteosarcomas arise in the head and neck. The mean age of these patients (35 y) is about 2 decades older than that of patients with osteosarcoma of the long bones. M:F = 1.5:1. Maxillary lesions arise from the sinus and alveolar ridge and usually produce no pain. Mandibular sarcomas occur in the body and ramus of the mandible and frequently cause a painful swelling.
Ewing sarcoma of sinonasal cavities is extremely rare. |
Chondrosarcoma |
Typically large, multilobulated, sharply demarcated soft tissue mass with heterogeneous, predominantly peripheral enhancement, when first detected, with rings and crescents of calcium or amorphous tumor matrix calcification. Bone changes include erosion and destruction. Periosteal reaction, if present, is usually mild. |
Sinonasal involvement is rare. These tumors occur in the wall of the maxillary sinus, at the junction of the nasal septum vomer with the sphenoid and ethmoid sinuses, and on the undersurface of the sphenoid bone. Most common in 30–45-y-old patients. |
Rhabdomyosarcoma
Fig. 7.17 |
Large, poorly defined, inhomogeneous soft tissue mass, with intense contrast enhancement, destroying adjacent bone. Tumors of the ethmoid sinuses have a marked propensity for crossing the skull base and intracranial extension. |
In adults, the ethmoid sinuses are the most common site of origin of head and neck rhabdomyosarcomas. Secondary sinonasal tumors may arise from adjacent orbital spread from orbital rhabdomyosarcoma or pharyngeal rhabdomyosarcoma. |
Non–Hodgkin lymphoma |
Lymphomas of the sinonasal cavities are often seen as relatively well-demarcated, polypoid, bulky masses of homogeneous soft tissue density; with moderate contrast enhancement, without calcification, with possible bone expansion. Lymphomas may also present as a poorly defined, sinus-obliterating soft tissue mass with bone erosion and destruction and surrounding infiltrations. |
Lymphomas arising in the nose and paranasal sinuses are of the non-Hodgkin type and are frequently observed in patients who have disseminated lymphoma or acquired immunodeficiency syndrome (AIDS). Nasal fossa and maxillary sinuses are the most common locations.
The disease is strongly associated with Epstein–Barr virus. It occurs mainly in Asian men, with a mean age of 50 y at the time of diagnosis. They can mimic the much more common entities of sinusitis, polyposis, granulomatous processes, and benign and malignant neoplasms. |
Idiopathic midline granuloma |
Destructive mass in the nasal septum leading to perforation. With progression, the disease spreads to the nose, face, hard and soft palates, adjacent sinuses, and orbits, with extensive bone destruction. |
This entity is now clearly defined as a T-cell lymphoma. |
Plasmacytoma |
Fairly well-defined soft tissue density mass confined to the soft tissues with moderate to marked contrast enhancement. These lesions tend to be expansile and are associated with bone remodeling, as well as bone erosion.
Multiple myeloma presents similarly to hematogenous metastases. It can have lytic lesions, which may also demonstrate diffuse osteopenia, and rarely sclerosis. |
Extramedullary plasmacytoma accounts for 4% of all nonepithelial tumors of the sinonasal cavities. Nasal cavity, nasal septum, sphenoidal sinus, and nasopharynx are the most common locations. |
Malignant melanoma |
Well-defined, polypoid or sessile, lobular soft tissue mass in the nasal cavity, with diffuse contrast enhancement. Calcifications may be seen. Associated bone destruction often is present that involves the maxillary antrum, ethmoids, or cribriform plate area. |
Less than 1% of all melanomas arise in the sinonasal cavities. Within the nasal cavity, the most common sites of melanomas are the anterior nasal septum, lateral nasal wall, and inferior turbinates. In the paranasal sinuses, the maxillary antrum is the site of origin in 80% of cases. Can be multicentric. |
Metastases |
The majority of metastases to the paranasal sinuses are to the bone. The tumor usually involves multiple sinuses, with bony wall destruction and extension into adjacent structures (e.g., intracranial and orbital). |
A tumor metastatic to the nasal cavity and paranasal sinuses is rare. Renal cell carcinoma accounts for the majority. Other primary sites are lung, breast, colon, stomach, prostate, and thyroid. The sites of involvement are maxillary sinus > ethmoid sinus > frontal sinus > nasal cavity > sphenoid sinus.
They occur in patients between 50 and 70 y of age. The clinical picture of metastatic lesions is similar to that of primary sinonasal malignancy. |
Craniofacial trauma |
Fracture |
Direct fracture signs on CT are fracture lines, discontinuity of bone, and displacement of bone.
Solitary strut (simple) fractures: Include limited fractures (unilateral or midline) of the orbital floor, medial orbital wall, isolated orbital rim, zygomatic arch, nasal arch, and localized sinus wall (frontal, maxillary).
Complex strut fractures: Limited fractures involving two adjacent anatomical areas: nasofrontal, nasomaxillary, nasoethmoidal, zygomaticomaxillary, and sphenotemporal. |
Maxillofacial fractures are common, often multiple, complex, and asymmetric. By approaching facial fractures in terms of the facial struts that are affected, these may be divided into three classes: limited, transfacial, and smash fractures. Traumatic midface injury often involves a combination of these fractures. |
Fig. 7.18a, b
Fig. 7.19
Fig. 7.20 |
Maxillary transfacial fractures: Require pterygoid plate fractures; can be divided into Le Fort I fracture, Le Fort II fracture, Le Fort II with I fractures, Le Fort II with zygomaticomaxillary complex fractures, Le Fort III fractures, Le Fort III with I fractures, and Le Fort III with II fractures. |
|
|
Smash fractures: Represent nasoethmoidal-orbital, central midface, and craniofacial fractures. Skull base fractures are present in a significant percentage of these patients. |
|
Fig. 7.21a, b |
Fracture of the zygomaticomaxillary complex: Term tripod fracture is misleading; extends through the four articulations of the zygomatic bone: zygomaticofrontal, zygomaticosphenoidal, zygomaticotemporal, and zygomaticomaxillary sutures with displacement and rotation of the zygoma. Additionally, this fracture may extend posteriorly to involve the pterygoid processes, greater sphenoid wing, and sphenotemporal buttress.
Nasoethmoidal-orbital fracture: Represents fractures of the lateral nasal bones, lower two thirds of the medial orbital rim, anterior ethmoidal structures, nasomaxillary buttress, and frontal process of the maxilla. Collapse of the nasoethmoidal complex and lateral displacement of the frontal process of the maxilla and medial orbital wall can result in a “blow-in” orbital fracture with traumatic telecanthus. |
|
Hemorrhage |
Diffuse or polypoid mucosal thickening, air–blood level, or complete air replacement by blood are manifestations of sinus bleeding. The density varies with the age of the blood. Acute clot manifests as a hyperdense mass. |
Occurs with trauma, surgery, neoplasm, vascular malformations, bleeding disorders, anticoagulation, and barotraumas (e.g., in divers and pilots of unpressurized aircraft). |
CSF leak |
A fracture through the inner table of the skull may be associated with a tear of the dura and arachnoid and a CSF leak. Frequently, the ethmoid, frontal, sphenoid, or petrous temporal bones may be involved. An air–fluid level in the adjacent paranasal sinuses might be due to blood or CSF. Pneumocephalus can occur in up to one third of all patients with posttraumatic or spontaneous CSF leak.
CT cisternography or radionuclide cisternography may be useful if high-resolution, thin-section axial and coronal cranial and facial CT and MR cisternography do not show the CSF fistula. |
Causes of CSF rhinorrhea include (1) blunt head trauma; (2) sequelae of skull base surgery; (3) destructive skull base lesions, including neoplasms (both benign and malignant) and empty sella; (4) developmental defects of the ethmoid, sphenoid, frontal, or petrous temporal bones with the formation of a meningocele or meningoencephalocele (with an intact tympanic membrane); and (5) fracture of the petrous temporal bone or other destructive processes in which CSF in the middle ear drains to the nose in the presence of an intact tympanic membrane. Head trauma accounts for 50% to 80% of all cases of CSF leak, and up to 16% are iatrogenic. Less than 5% of all cases of CSF rhinorrhea are spontaneous.
Most cases of CSF rhinorrhea begin soon after a head injury and cease spontaneously within 7 to 180 days. Rhinorrhea may occur intermittently and can increase on bending forward, with Valsalva maneuver or jugular vein compression. CSF leak occurs often without nasal congestion, sneezing, lacrimation, or aural discharge. |
Miscellaneous |
Rhinolith and sinolith
Fig. 7.22 |
Nasal or sinus mass. May be heavily calcified. The calcification appears as a cast surrounded by soft tissue related to inflammatory reactions. |
Foreign body in sinonasal cavity acting as nidus may become encrusted with mineral salts when retained for a long period. Very rare.
Rhinoliths may produce nasal obstruction, a malodorous nasal discharge with local pain, and epistaxis. |
Pneumosinus dilatans |
Focal or diffuse abnormal expansion and asymmetric dilation of a paranasal sinus with normal thickness of the displaced walls. The frontal sinus is most often affected, followed by the ethmoid and sphenoid sinuses. |
Rare condition. Can occur separately or in association with meningioma, mucocele, fibrous dysplasia, acromegaly, arachnoid cyst, and cerebral hemiatrophy.
A hypersinus is an enlarged paranasal sinus that does not expand the surrounding bone beyond its normal contours and has bony walls that are of normal thickness. Pneumoceles are hyperaerated paranasal sinuses or air cells associated with focal or generalized luminal enlargement and focal or diffuse thinning of adjacent bony wall. Pneumatoceles are extraosseous gas collections that usually form after trauma, infection, or surgery. |