7 Nonvariceal Upper Gastrointestinal Hemorrhage
Eric J. Monroe and Karim Valji
7.1 Introduction
Selective arteriography of the gastrointestinal tract for enteric hemorrhage was an early mainstay in the nascent field of interventional radiology half a century ago. 1 , 2 Ingenious angiographers soon made the not-so-obvious leap to using this technique for transcatheter management. 3 As the technology and experience with this technique expanded, therapeutic options also evolved, progressing from infusion of vasoconstrictive agents to deployment of a wide range of embolic agents. Mesenteric angiography and intervention soon became a common request for patients with ongoing gastrointestinal bleeding.
As in other arenas of medicine, the multidisciplinary approach to gastrointestinal hemorrhage has evolved substantially in recent decades. Endoscopists have greatly expanded their diagnostic and therapeutic capabilities in this area. Medical therapies also continue to improve, with a wide range of acid-reducing agents and selective cyclooxygenase inhibitors now available. The discovery of and treatment for Helicobacter pylori have altered the epidemiology of peptic ulcer disease. 4 However, advances in medical therapy have not convincingly decreased mortality. 5 Gastrointestinal hemorrhage remains a common clinical problem, with an estimated yearly incidence of 100 to 300 per 100,000 people. 6 , 7 , 8 This condition also continues to be a leading cause of emergency room visits and hospital admissions, with mortality as high as 10%. 6 , 9 , 10 Cases refractory to initial treatment are also common. For these reasons, angiography with catheter-directed therapy remains a critical tool in the management of nonvariceal upper gastrointestinal hemorrhage. 11 , 12
7.2 Patient Presentation
Upper gastrointestinal bleeding is defined as bleeding originating from the esophagus to the ligament of Treitz and is far more common than lower gastrointestinal bleeding. 13 Transpapillary bleeding from hepatic and pancreatic sources enters the duodenum and therefore presents as upper gastrointestinal hemorrhage. A wide range of clinical presentations may be encountered, largely dependent on the rate of bleeding. If blood losses have been greater than 500 mL in 24 hours, hemodynamic perturbations such as shock may be present. Conversely, blood losses of less than 100 mL in 24 hours may be asymptomatic and incidentally discovered as anemia.
Hematemesis and bloody aspirate from a nasogastric tube are compelling signs of an upper gastrointestinal source, whereas hematochezia generally suggests a lower source. Melena may result from bleeding anywhere along the length of the gastrointestinal tract, depending on the rate of hemorrhage. The orifice of bleeding alone is an unreliable indicator of pathology. 6 In approximately 10% of cases, brisk bleeding from an upper intestinal tract source may present with bright red blood through the rectum.
A careful review of past medical and surgical history and medication use may favor one source over another. Nonenteric sources of bleeding such as epistaxis or hemoptysis should be reasonably excluded.
7.3 Etiologies
Peptic ulcer disease remains the most common underlying condition causing upper gastrointestinal hemorrhage, manifesting as erosive esophagitis, erosive gastritis, or ulcers of the stomach or duodenum. These manifestations of peptic ulcer disease account for approximately 70% of upper gastrointestinal bleeds. 14 Potentiating factors include H. pylori infection, use of nonsteroidal anti-inflammatory medications, and alcohol consumption.
Variceal bleeding and portal hypertensive gastropathy comprise the next largest fraction of cases and are discussed elsewhere in this text.
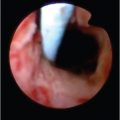
Mallory–Weiss lesions are focal tears of the distal esophagus and gastroesophageal junction involving the mucosa and underlying vasculature. 15 Bleeding is classically preceded by retching, often associated with alcohol consumption. In the absence of concurrent gastroesophageal variceal bleeding, most of these cases can be managed conservatively. Infrequently, these lesions require endoscopic and/or transcatheter intervention (▶ Fig. 7.1).

Dieulafoy lesions are focal erosions of an otherwise normal submucosal artery found most commonly in the proximal stomach but potentially anywhere along the length of the gastrointestinal tract. 16 Risk factors include antiplatelet agent use and alcohol consumption. Most lesions identified at endoscopy are successfully managed in that setting 17 ; however, bleeding may be intermittent and endoscopically occult.
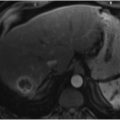
Both benign and malignant gastrointestinal tumors have the potential to cause gastrointestinal hemorrhage if there is erosion across the mucosal surface. Bleeding in such cases is typically slow and subclinical but may be profound (▶ Fig. 7.2).

Transpapillary bleeding may result from pathology of the liver, gallbladder, or pancreas. Bleeding localized to the pancreatic duct is termed hemosuccus pancreaticus and is typically associated with chronic pancreatitis or pseudocysts. 18 Postpancreatitis pseudoaneurysm of the pancreatic arteries, splenic artery, or gastroduodenal artery and subsequent bleeding may be either retroperitoneal or transpapillary. Less commonly, fistulous connections to the stomach, either spontaneous (▶ Fig. 7.3) or iatrogenic (▶ Fig. 7.4), can lead to postpancreatitis gastric bleeding. Hepatic biliary sources are frequently iatrogenic as a result of biopsy or transhepatic biliary drain placement (▶ Fig. 7.5).



An aortoenteric fistula is a rare but life-threatening entity that often results in patient death before further workup can be performed. Although any abnormal fistulous connection between the aorta or branch vessel and the digestive tract meets the broad definition of an aortoenteric fistula, most occur between the transverse duodenum and abdominal aorta. Primary aortoenteric fistulas are most commonly associated with abdominal aortic aneurysms but may also arise as a result of malignancy, peptic ulcer disease, trauma, infection, inflammatory bowel disease, or foreign body ingestion. 19 Secondary aortoenteric fistulas occurring after aortic aneurysm repair or para-aortic surgery are more common. Left untreated, this condition is frequently fatal.
7.4 Initial Management
Patients presenting with hemodynamic instability or obvious active bleeding require admission to the intensive care unit. Management begins with establishment of large-bore intravenous (IV) access followed by resuscitation with crystalloid fluids and/or blood products. 20 A nasogastric tube should be placed, along with an endotracheal tube if airway protection is needed. Nasogastric lavage returning bloody aspirate all but confirms a proximal source of bleeding. A lavage without evidence of bleeding does not exclude an upper gastrointestinal source, however. Approximately 15% of such patients will have high-risk lesions identified at endoscopy. 21
Reversal of anticoagulation and correction of thrombocytopenia or coagulopathy (when present) should be performed. Empiric therapy with high-dose proton-pump inhibitors should be initiated and continued until the need for acid blockade is excluded. Splanchnic vasoconstrictors such as vasopressin may be initiated to support systemic pressure while decreasing enteric blood flow. Similarly, empiric therapy with a somatostatin analogue (octreotide) may address potential portal hypertensive bleeding but may also reduce the risk of bleeding from nonvariceal etiologies. 22 A wide variety of tests are available to diagnose H. pylori, which may then be eradicated with multidrug antibiotic therapy. With medical support established, management can proceed to bleeding localization.
7.5 Diagnosis
7.5.1 Endoscopy
Upper endoscopy is the diagnostic modality of choice for upper gastrointestinal hemorrhage 23 , 24 ; this technique reduces the rate of rebleeding, the need for surgery, and patient mortality compared to medical management. 25 Although many cases of hemorrhage will resolve spontaneously, early endoscopy (within 24 hours) is advocated by many to localize, treat, and prevent the recurrence of bleeding. 23 With this procedure, arterial and portal hypertensive etiologies of bleeding can be readily distinguished. Details of the endoscopic approach are beyond the scope of this text. In general, current prevailing techniques for identifying and treating hemorrhage include ulcer bed irrigation, epinephrine injection, use of clips, thermocoagulation, and sclerosant injection. 4 Despite high initial technical success rates with these techniques, rebleeding occurs in 15 to 20% of cases. 4 Complications of endoscopy include mucosal trauma, aspiration, bowel perforation, and induction of uncontrollable bleeding. Clip localization for uncontrollable bleeding can aid in subsequent angiographic intervention.
7.5.2 Noninvasive Imaging
Nuclear Medicine
Because of the logistics of the examination and image acquisition, scintigraphy is largely reserved for hemodynamically stable patients. This technique remains an invaluable tool for cases of intermittent and/or obscure bleeding, however. The prevailing scintigraphic technique for the evaluation of gastrointestinal bleeding involves autologous red blood cell labeling with technetium-99 m, reinfusion, and subsequent periodic imaging of the abdomen. With this procedure, bleeding rates as low as 0.1 mL/min can be detected. 26 The relatively long half-life of technetium-99 m also allows for delayed imaging and identification of intermittent bleeding occurring over the course of the examination window.
These examinations suffer from poor spatial localization and are not effective at characterizing underlying pathology, but they have the potential to decrease the rate of negative angiograms when used appropriately. 27 Single-photon emission computed tomography (SPECT)/CT offers more conspicuous localization of bleeding compared to planar imaging alone, 28 and so the use of this modality is increasing.
CT Angiography
Similar to scintigraphy, CT angiography has been historically reserved for hemodynamically stable patients with upper gastrointestinal bleeding. As sensitivity for the detection of bleeding has increased, CT has been increasingly applied for the workup of gastrointestinal bleeding. Recent studies have yielded exciting results, particularly in the context of brisk and lower gastrointestinal bleeding. 29 , 30 , 31 A recent meta-analysis found CT angiography to be both cost effective and accurate in the detection and localization of gastrointestinal bleeding. 32 Detection of bleeding rates as low as 0.3 mL/min has been demonstrated in an animal model. 33 The seemingly ever-increasing rapidity and quality of CT angiography will almost certainly lead to an increasing role for this modality in the workup of upper gastrointestinal hemorrhage; CT angiography has already supplanted scintigraphy at some institutions. At the authors’ institution, the CT angiography protocol for gastrointestinal bleeding includes negative oral contrast and a 100- to 150-mL IV contrast bolus with three-phase acquisition (noncontrast, arterial, and 120-second delay).
7.6 Anatomy
Hemorrhage in the upper gastrointestinal tract originates from branches of either the celiac artery or superior mesenteric artery (SMA). Variation in the mesenteric arterial vasculature is common. 34 The celiac artery arises from the anterior surface of the aorta at the T12–L1 level and classically gives rise to the common hepatic artery, left gastric artery, and splenic artery. Each of these vessels may anomalously originate directly from the aorta; this is most frequently seen with the left gastric artery. 35 The SMA arises from the anterior surface of the aorta approximately 1 cm below the celiac artery. SMA supply to the upper gastrointestinal tract typically occurs via the inferior pancreaticoduodenal artery. The common hepatic artery is replaced to the SMA in approximately 5% of individuals. 36 In less than 1%, the celiac artery and SMA share a common origin from the aorta (▶ Fig. 7.6), termed either the celiomesenteric artery or the celiacomesenteric artery. 37 Rarely, aberrant regression of the embryologic ventral anastomoses leaves a direct connection between the celiac artery and SMA, termed the arc of Buhler.

The stomach is richly perfused from the paired gastric arteries, paired gastroepiploic arteries, and short gastric arteries. 38 , 39 , 40 The left gastric artery supplies the upper aspects of the lesser curvature and cardia. The right gastric artery collateralizes with the left to supply the lower aspects of the lesser curvature and antrum. The right gastric artery arises from the proper hepatic artery in approximately half of individuals. Variant origins, in decreasing order of frequency, include the left hepatic artery, gastroduodenal artery, common hepatic artery, right hepatic artery, and middle hepatic artery. 41 The right gastroepiploic artery is the terminal branch of the gastroduodenal artery and supplies the greater curvature of the stomach. The short gastric and left gastroepiploic arteries arise from the splenic artery to supply the fundus and lateral aspects of the greater curvature, respectively. This rich and redundant blood supply of the stomach allows for safe empiric embolization in cases of angiographically occult bleeding. However, brisk gastric bleeds may need to be approached from several vessels as collateral flow perfuses the culprit vessel (▶ Fig. 7.7).

Arterial supply of the duodenum can be roughly approximated by the duodenal segment. 42 The duodenal bulb is perfused by branches of the right gastric and right gastroepiploic arteries. Named branches of the gastroduodenal artery to this region include the supraduodenal artery and retroduodenal artery; the latter is also called the posterior pancreaticoduodenal arcade. The superior pancreaticoduodenal arterial branches supply the lower aspects of the bulb to the midaspect of the descending duodenum. The remaining duodenum receives blood primarily via the inferior pancreaticoduodenal arterial branches.
The pancreatic arterial anatomy must also be briefly described because of its role in transpapillary bleeding. The superior and inferior pancreaticoduodenal arteries perfuse the head and uncinate process. Typically, the largest of the pancreatic arteries, the dorsal pancreatic artery, has a highly variable origin anywhere posterior to the pancreatic neck. The distal body and tail are supplied by the pancreatic magna and caudal pancreatic arteries, which arise from the mid- and distal splenic artery, respectively. Each of these vessels may collateralize via the transverse pancreatic artery, which follows the long axis of the pancreatic body and tail. 43
The rich and redundant supply of the upper abdominal visceral organs permits robust collateralization in cases of severe narrowing or occlusion due to atherosclerosis, prior surgery, or other causes. In such cases, a “backdoor” approach to catheterizing a given vascular territory may be required (▶ Fig. 7.8).

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree